Manual of Patent Office Practice (MOPOP)
Chapter 1 Introduction to Patents and the Patent Office
1.01 Purpose of the Manual of Patent Office Practice
1.03 CIPO and the Patent Office
1.04 Where to get more information
Chapter 2 Communicating with the Patent Office
2.01 Introduction to communicating with the Patent Office
2.02 Part 1 – Written communications
2.02.01 General requirements for submission of written communications
2.02.02 General formatting requirements
2.02.02a Exception for communications relating to more than one application or patent
2.02.02b Exception identifying the patent application number
2.02.03 Who can communicate with the Patent Office
2.02.05 Physical delivery of written communications
2.02.05a Regular mail or in person
2.02.05b Designated establishment – Registered MailTM and XpresspostTM services of Canada Post
2.02.06 Submission of documents, information and fees by electronic means
2.02.06c(i) PCT international correspondence from CIPO
2.02.07 Submission of documents, information or fees on electronic media
2.02.07a Acceptable electronic formats
2.02.08 Electronic form of sequence listings
2.02.08a Canada as receiving Office under the PCT: Electronic filing of sequence listings
2.02.09 Written communications from the Commissioner of Patents or the Patent Office
2.02.09a Practice for returned communications
2.02.09d Practice for allegations of delayed receipt of examiner’s report from the Patent Office
2.02.09e Exception – written communications sent before suspension, revocation or surrender
2.03.01 Date of receipt accorded to documents, information or fees submitted to the Patent Office
2.03.01a Date of receipt – physical delivery to Patent Office
2.03.01b Date of receipt – physical delivery to designated establishments
2.03.01c Date of receipt – submission by electronic means
2.03.02a Calculation of time limits
2.03.03a Time period extended for prescribed and designated days
2.03.03c Unexpected closures of the office
2.03.03d Designated days – force majeure
2.03.03e Requests for extensions of time – subsection 3(1) of the Patent Rules
2.03.03e(i) Extension of Time for Examiner Requisitions
2.03.03e(ii) Extension of Time – Delayed Receipt of Examiner Requisitions
2.03.03f Non-application of subsection 3(1) of the Patent Rules
2.03.03g Extension of time to ‘top-up’ small entity fees – subsection 3(3) of the Patent Rules
2.03.03i Extension of time to ‘top-up’ – erroneous information – subsection 3(4) of the Patent Rules
2.03.04 Time Limits for PCT national phase applications before national phase entry
Chapter 3 Filing a Patent Application
3.01 Introduction – Types of patent applications
3.02 Filing a regular Canadian patent application
3.02.01 Requirements to obtain a filing date for a regular Canadian patent application
3.02.02 Failure to provide all of the information and/or documents to secure a filing date
3.02.03 Description provided in a language other than English or French
3.02.04 Reference to a previously filed application in place of a description
3.02.04b Copy of the previously filed application
3.02.04c Translation of the previously filed application if not in English or French
3.02.05 Addition to specification or addition of drawing
3.02.05a Time period for addition
3.02.05b Effect on filing date
3.02.07 Numbering of applications
3.02.09 Withdrawal of an application
3.03 PCT national phase applications
3.04.01 Meaning of "original application"
3.04.02 Filing requirements for divisional applications
3.04.02a Original patent application number submitted after presentation date
3.04.03 Specification and drawings submitted on the presentation date
3.04.04 Application fee for divisional application
3.04.05 Maintenance fees for divisional application
3.04.07 Deadline for filing a divisional application
3.04.09 Notices in respect of original application
3.04.10 Public inspection of divisional application
Chapter 4 Compliance Requirements
4.01 Introduction – Compliant Patent Applications
4.01.01 Compliant PCT National Phase Applications
4.02.01 Notice of non-compliance – response to notice
4.04 Inventor information and establishing entitlement
4.07 Translation of a description or previously filed application
4.08 Notice requiring a translation of the description – subsection 15(4) of the Patent Rules
4.09 General formatting requirements for parts of application
5.02 Register of Patent Agents
5.02.01 Updates to patent agent addresses
5.02.02 Registered foreign practitioners
5.03.01 Appointment of common representative – filing date or PCT national phase entry date
5.03.02 Appointment of common representative by notice
5.03.03 Common representative by default – applications
5.03.04 Common representative by default – patents
5.03.05 Common representative by default in case of transfers (applications and patents)
5.04.01 Requirement to appoint a patent agent
5.04.02 Commissioner’s Notice – Requirement to appoint a patent agent
5.04.03 Appointment of patent agents
5.04.03a Appointment of patent agent in the petition or in the request for PCT national phase entry
5.04.03b Appointment of patent agent by notice
5.04.03c Consent of the patent agent to the appointment
5.04.04 Default appointment of patent agent – patents
5.04.05 Default appointment of patent agent – transfers
5.04.06 Revocation of appointment of patent agent
5.04.07 Deemed appointments and revocations for members of firms (appointed patent agent)
5.05.01 Appointment of the associate patent agent
5.05.01b Appointment of associate patent agent by notice
5.05.02 Default appointment of associate patent agent – patents
5.05.03 Revocation of appointment of associate patent agent
5.05.04 Deemed appointments and revocations for members of firms (appointed associate patent agent)
5.06 Succession of patent agent
5.07 Representation Requirements – what actions can be taken by whom
5.07.01 Representation during prosecution of the patent application
5.07.01a Representation by others
5.07.02 List of actions and persons authorized to represent – patent applications
5.07.02b Payment of annual maintenance fees – applications
5.07.02c Payment of other fees relating to an application
5.07.02d Signing of small entity declaration
5.07.02e Reinstatement of an application deemed abandoned for failure to pay maintenance fee
5.07.02f Submission of request to record a transfer
5.07.02g Submission of request to record a name change
5.07.02i Interview with patent examiner
5.07.02j Correction of applicant identity
5.07.02k Representation of Patent Applicants – who can act?
5.07.03 Representation for procedures relating to patents
5.07.03a Payment of annual maintenance fees – patents
5.07.03b Submission of request to record a transfer
5.07.03c Additional term, reissue, disclaimer and re-examination
5.07.03d Signing of small entity declaration
5.08 Default Correspondent – Who will the Patent Office correspond with
5.09 Disregarded communication
5.09.01 Applicant or Patentee who is not the common representative
5.09.02 Patent agent not appointed
5.09.03 Patent agent’s name not provided
Chapter 6 Ownership, Inventorship, Transfers, Changes of Names
6.01 Ownership – applicants/patentees
6.01.02 Maintaining chain of title
6.01.03 Adding and removing applicants
6.01.04 Jurisdiction of the Federal Court
6.02.01 Adding and removing inventors (patent application)
6.03 Correcting errors in the naming of an applicant
6.03.01 Correction of errors in naming the applicant - identity vs name
6.03.02 Correction of applicant identity
6.03.02b Time limit to submit correction of applicant identity – PCT national phase application
6.03.02c Correction of applicant name (no change in identity)
6.03.04 Effect of correction of an error in a patent application
6.04 Correcting errors in the naming of an inventor – identity vs name
6.04.01 Correction of inventor identity
6.04.02 Correction of inventor name (no change in identity)
6.04.03 Content of request to correct inventor name and/or identity in a patent application
6.04.04 Effect of correction of inventor name and/or identity in a patent application
6.05 Transfers, Changes of Name, Registration of Documents
6.06.01 Right or Interest in an Invention
6.06.02 International Applications
6.06.03 Request to record a transfer
6.06.03a Request to record a transfer by applicant or patentee
6.06.03b Request to record a transfer by the transferee
6.06.05 Removal of transfer recording
6.07 Changes of name (applicants)
6.07.01 Change of Name of applicant or patentee
6.07.02 Request to record a name change
6.07.03 Certificate of a name change
6.08 Registration of related documents
6.08.01 Request to register a document
6.08.02 Certificate of registration of a document
6.10 Protect your privacy on the Canadian Patents Database (CPD)
7.03.01 Making the request for priority
7.03.01a Exception – priority application number is not known
7.03.02 Time period for the request for priority
7.03.03 Correction of priority information – priority filing date
7.03.04 Correction of priority information – priority filing country/office or number
7.04 Copy of priority application
7.04.01 Manner of Submission of Certified Copy
7.04.02 Digital library – WIPO Digital Access Service
7.04.03 Exception – copy of priority application not required
7.04.04 Time period to submit copy of priority documents
7.04.05 Commissioner’s Notice to submit copy of priority documents
7.04.06 Exception – copy of the priority document not available
7.05 Translation of priority document
7.06 Restoration of the right of priority
7.06.01 Restoration of the right of priority – effective in Canada
7.06.02 Request to restore the right of priority
7.06.03 Time period for request for restoration of right of priority
7.06.04 Right of priority deemed restored
7.06.04a Deemed restoration of right of priority – effective in Canada
7.07 Considered withdrawal of a request for priority
7.08 Withdrawal of a request for priority
7.09 Applications filed before an intergovernmental authority
7.10 Applications filed before an international organisation
7.11 Applications filed before the PCT
7.12 Applications filed before the European Patent Office
7.13 Extensions of time not permissible
7.14 Time period extended for prescribed and designated days
7.15 Special topics related to priority
7.15.02 Types of recognised priority documents
8.01 Maintenance fees for patent applications
8.01.01 Amounts and due dates for maintenance fees for patent applications
8.01.02 Late fee period – maintenance fees for patent applications
8.01.03 Maintenance fees for divisional applications
8.01.04 Maintenance fees for PCT national phase entry applications
8.02.01 Public inspection of PCT national phase applications
8.02.02 Early public inspection
8.02.03 Confidentiality of applications not yet open to public inspection
8.02.04 Applications open to public inspection
8.02.05 Canadian Patents Database
8.02.05a Protecting your privacy – personal information in applications, patents and documents
8.02.06 Publication of lists of granted patents and patent applications open for public inspection
8.03 Withdrawal of patent applications
8.03.01 Effect of Withdrawal on Applications Being Open to Public Inspection
8.03.02 Effect of Withdrawal of priority on opening to public inspection
8.04 Cover Page Publication and Corrections
Chapter 9 Abandonment and Reinstatement of Patent Applications, Third Party Rights
9.02 Abandonment of patent applications
9.02.01 Deemed abandonment of patent applications under subsection 73(1) of the Patent Act
9.02.02 Deemed abandonment of patent applications under subsection 73(2) of the Patent Act
9.02.03 Courtesy letters of abandonment
9.03 Reinstatement of abandoned patent applications
9.03.02 Reinstatements Requiring Determination of Due Care
9.03.03 Time Period for Reinstatement
9.03.04 Single Request for Reinstatement for Multiple Abandonments
9.04.01 Transitional Provisions
9.04.02 Determination of due care by the Commissioner of Patents
9.04.06 Recommended information to include with the request
9.04.07 Office procedure – determination
9.04.08 Office procedure – observations
9.04.09 Office procedure – service standard
10.01.02b Request for examination
10.01.02c Exception – Amount due: International application fees
10.02.01 Definition of small entity
10.02.02 Small entity declaration
10.03.03 General authorization statements
10.04.01 Date of Request for Examination – insufficient excess claims fee and subsequent amendment
10.06.01 Waiver of fees – correction or reissue request
10.06.02 Waiver of fees – Extension of Time - Delayed Receipt of Examiner’s Report
10.07 Client Service Standards
Chapter 11 Administrative Practice of Examination
11.01.02 What is the time limit to request examination?
11.01.03 Late fee period – request for examination is not made within the prescribed time limit
11.03.01 Advancing examination (“special order”)
11.03.02 Applications related to green technology
11.03.03 The Patent Prosecution Highway (PPH)
11.04.02a Requesting Continued Examination after initial request for examination
11.04.02b Requesting Continued Examination – Subsequent Requests
11.05 Amendments of Patent Applications
11.05.02 Amendments to PCT applications
11.05.03 Amendments in response to an examiner’s report or notice
11.05.04 Claim amendment deemed not included
11.05.05 Corrections to translations
11.05.06 Format and requirements for submitting amendments
11.05.06a Statement of purpose of amendment and page replacement instructions
11.05.06b Cover letter for amendments
11.05.07 Other submissions related to the application accompanying the amendment
11.06 Suspension of examination
Chapter 12 Fundamentals of Examination
12.01.01 Examination of the abstract, description and drawings
12.02 Examination of the claims using purposive construction
12.02.01 Steps of purposive construction
12.02.02 Considerations for claim construction
12.02.02a Use a fair, balanced and informed approach
12.02.02b Identify the person skilled in the art
12.02.02c Identify the common general knowledge
12.02.02d Identify the problem and solution
12.02.02e Determine which elements of the claim solve the identified problem
12.02.03 Examination once the claims have been construed
12.02.04 Examples of purposive construction
12.04.01 Requisitions concerning foreign applications
12.04.02 Biological Deposit requisitions
12.04.03 Withdrawal of an examiner’s report
12.04.03a Minor errors in an examiner’s report
12.05 Other Notices During Examination
12.05.02 Correction of national phase translation
12.05.03 Translation of Priority Documents
12.05.04 Accessibility of Priority Documents
12.05.05 Notice requiring a request for continued examination
12.06.01 Applicant-initiated interviews
12.06.02 Examiner-initiated interviews
13.02 Reference characters in abstracts
13.03 Examination of abstracts
14.02 General requirements of disclosure
14.02.02 Addressee is the person skilled in the art
14.02.03 Description supplemented by common knowledge
14.02.04 Misleading or erroneous statements
14.02.05 Addressee not to be presented with problems to solve
14.02.06 Theory of the invention
14.03 Disclosing a solution to a practical problem
14.04 This section has been intentionally left blank
14.04.01 This section has been intentionally left blank
14.04.02 This section has been intentionally left blank
14.05.01 Functional limitations
14.05.02 Disclosure of biotechnological inventions
14.05.03 The applicant as their own lexicographer
14.05.04 Disclosure of trademarked products
14.05.05 Description by reference to the claims
14.05.06 Statements expanding the scope of the claims
14.05.07 References to foreign practice or law
14.07 Formalities requirements of the description
14.07.01 Pages of the description
14.07.02 Drawings, graphics and tables
14.07.03 Identification of trademarks
14.07.04 Identification of documents
14.08 Amendments to the description
14.09 Office actions on the description
15.01.01 Amendments to drawings
16.02 Principles of construction
16.05.01 Claims referring to description or drawings
16.05.02 Scope in relation to description
16.05.03 Ranges not specifically described
16.06 Dependent claims and formalities requirements
16.06.01a Transitional Considerations
16.07.01 Exhaustive combinations
16.08.01 Product-by-process claims
16.10 Process, method, method of use and use claims
16.10.01 Process and method claims
16.10.02 Method of use and use claims
Chapter 17 Statutory Subject-Matter
17.01 Statutory subject-matter
17.01.05 Composition of matter
17.02 Inventions must not be disembodied
17.03.01 Scientific principles and abstract theorems
17.03.02 Methods of medical treatment or surgery
17.03.05 Features of solely intellectual or aesthetic significance
17.03.08 Schemes, plans, rules, and mental processes
Chapter 18 Anticipation, Obviousness and Double-Patenting
18.01.01 Prior art when assessing anticipation
18.01.01b Third party anticipation
18.01.01c First-to-file anticipation based on filing-date
18.01.01d First-to-file anticipation based on priority date
18.01.02 Assessing anticipation
18.01.03 Anticipation by prior sale or use
18.01.04 Implicit or inherent disclosure
18.01.05 Anticipation based on related teachings
18.02.01 Prior art when assessing obviousness
18.02.01a Obviousness and prior disclosures by the applicant
18.02.01b Obviousness and third party disclosures
18.02.02 Assessing obviousness
18.02.02a Person skilled in the art (Step 1(a))
18.02.02b Common general knowledge (Step 1(b))
18.02.02c Identifying the inventive concept (Step 2)
18.02.02e Do the differences constitute an inventive step? (Step 4)
18.02.03 Obvious to try considerations
18.02.05 Obviousness and utility
18.02.06 Obviousness of anticipated claims
18.03.01 Claim date based on multiple previously filed applications
18.03.01a Same subject-matter in multiple previously filed applications
18.03.02 U.S. continuation and continuation-in-part applications
18.05 Establishing the publication date of prior art
18.05.01 Verifying the content of priority documents
18.05.01a Requesting translations of priority documents
18.05.01b Transitional consideration
18.06.03 Co-pending applications
18.06.04 Division at the direction of the Office
19.01.01 Controllability and reproducibility
19.01.02 Demonstration or sound prediction
19.01.03 Requirements for sound prediction
19.01.03b Sound line of reasoning
19.01.03c Proper disclosure of the sound prediction
19.02 Office actions on utility
20.03 Applications originally filed in a language other than English or French
20.03.01 Regularly-filed Canadian (non-PCT) applications
20.03.02 PCT national phase applications
20.03.02a Corrections to translations of PCT national phase applications
20.04.01 New matter when filing a divisional application
20.04.01a Regularly-filed Canadian (non-PCT) applications
20.04.01b PCT national phase applications
20.04.02 New matter when amending a divisional application
20.04.02a PCT national phase applications
20.05 Transitional considerations
21.03 Meaning of “one invention only”
21.04 Canadian unity standard harmonious with PCT standard
21.05 General inventive concept
21.06 A priori and a posteriori evaluation
21.07 Examining for unity of invention
21.07.01 Content of the report
21.07.02 Explaining a lack of unity defect
21.07.03 When a lack of unity defect can be identified
21.07.04 Responding to a requisition
21.07.05 Election of an invention
21.07.06 Referral to the Commissioner of Patents
21.08.01 Claims in different categories of invention
21.08.02 Unity without a claim to the inventive linking feature
21.08.03 Unity of invention and utility
21.08.04 Markush groups and lists of alternatives
21.08.05 Intermediates and final products
21.08.06 Multi-step methods of preparation
21.09 Right to file a divisional application
21.10 Examination of divisional applications
Chapter 22 Computer-Implemented Inventions
22.02.05 Composition of matter
22.03 Examining computer claims
22.03.01 Adapting a computer to solve a problem
22.03.02 Patentability and programming
22.05.01 Written description and enablement
22.05.02 Source code or pseudocode
22.05.03 Common general knowledge and programming
22.06.01 Anticipation by prior use
22.08.01 Computer-implemented method claims
22.08.04 Software product claims
22.08.05 Means statements in claims
22.09.01 Graphical user interfaces
22.09.04 Computer-Aided Design (CAD) Programs
Chapter 23 Biotechnology and Medicinal Inventions
23.02.01 Higher and lower life forms
23.02.03 Processes to produce life forms
23.03 Medical methods and uses
23.03.01 Medical and surgical methods
23.03.02 This section has been left intentionally blank
23.03.03a Claims of indefinite scope or lacking clarity
23.03.04 Medical diagnostic methods
23.03.04a Identifying the problem
23.03.04b Determining the solution to the identified problem
23.03.04c Purposive construction
23.03.04d Determining whether a claim defines statutory subject-matter
23.04 Sufficiency of the description
23.05 Nucleic acids and proteins
23.05.01 Defining by structure
23.05.02 Defining by functional limitation
23.05.03 Nucleic acid and amino acid terminology
23.05.04 Hybridizing nucleic acids
23.05.05 Sequence alignment methods
23.05.06 Considerations respecting obviousness
23.05.07a Requirements for a sequence listing
23.05.07b The PCT sequence listing standard
23.05.07c Representation of sequences
23.05.07d Identification of a sequence listing
23.05.07e Variable/ambiguity symbols and special situations in a sequence listing
23.05.07f Correction of a sequence listing
23.06 Deposits of biological materials
23.06.01 Considerations respecting sufficiency of disclosure
23.06.02 Considerations respecting anticipation
23.07.01 Polyclonal antibodies
23.07.02 Monoclonal antibodies
23.07.02a Sufficiency of the disclosure
23.07.02b Other patentability requirements
23.07.03 Humanized and chimeric monoclonal antibodies
23.07.04 Fully human monoclonal antibodies
23.07.05 Antibodies and utility
23.08 Synergistic chemical combinations
23.10 Appendix 1 Deposits of biological material
23.10.02 Where to make a deposit
23.10.03 When to make a deposit
23.10.04 Identifying a deposit
23.10.06 New and substitute deposits
23.10.07 Access to deposited biological material
23.10.08 Nomination of an independent expert
23.11 Appendix 2 Steps for obtaining samples of biological materials
Chapter 24 Protests and filings of prior art prior to grant
24.03 Applying protests or filings of prior art
Chapter 25 Allowance, Final Fee and Issuance of Patents
25.01 Allowance and notice of allowance
25.01.01 Conditional allowance and conditional notice of allowance
25.01.02 Amendments after notice of allowance or conditional notice of allowance
25.01.03 Exception – amendment of an obvious error
25.02 Withdrawal of notice of allowance or conditional notice of allowance
25.02.01 Withdrawal by the Commissioner – Notice of allowance
25.02.02 Withdrawal by the Commissioner – Conditional notice of allowance
25.03 Issuance of a patent on payment of final fee
Chapter 26 Final Actions and Post-Rejection Practice
26.03 Examination before a rejection
26.04 Rejecting an application
26.04.01 The Final Action Report
26.05 Responses to a Final Action
26.05.01 Responses that overcome the rejection
26.05.02 Responses that do not overcome the rejection
26.07 Review of a rejected application
26.07.01 Referral to the Patent Appeal Board
26.07.02 Communication with the applicant
26.07.03 Issues arising during the review process
26.07.03a Clarification of certain matters
26.07.04 Opportunity to be heard
26.07.05 Decisions without a hearing
26.07.06 Recommendation to the Commissioner
26.08 The Commissioner’s Decision
26.08.01 Rejection not justified and application allowable
26.08.03 Amendments required by the Commissioner
26.09 Appeals of Commissioner’s Decisions
26.10 Prosecution following a decision of the Court
Chapter 27 Patent Maintenance Fees, Deemed Expiry and Reversal of Deemed Expiry
27.01 Maintenance fees for patents
27.01.01 Amounts and due dates for maintenance fees for patents
27.01.01a Exception – patent granted with outstanding maintenance fee for patent application
27.01.02 Late and non-payment of patent maintenance fees
27.02 Deemed expiry of patents and additional term
27.02.01 Courtesy letters of deemed expiry of patents
27.02.02 Reversal of deemed expiry of patents
27.02.03 Time period for reversal of deemed expiry
27.03.01 Transitional Provisions
27.03.02 Determination of due care by the Commissioner of Patents
27.03.03 The due care standard
27.03.05 Recommended information to include with the request
27.03.06 Office procedure – determination
27.03.07 Office procedure – observations
27.03.08 Office procedure – service standard
Chapter 28 Corrections to Granted Patents
28.02 Obvious errors made by the Commissioner
28.03 Obvious errors made by the re-examination board
28.04 Error in the naming of a patentee or inventor
28.05 Obvious error in the specifications or drawings
28.06 Content of request to correct errors in a patent
28.07 Effect of error correction in a patent
29.01.02 The roles of the Patent Office and the Courts
29.01.03 Effect of a disclaimer
30.01.03 Second stage of re-examination
30.01.04 Completion of re-examination
30.01.05 Effect of the re-examination certificate
30.01.06 Appeals from re-examination
31.01.01 Time limit for filing an application for reissue
31.01.02 Patent must be “defective or inoperative”
31.01.02a The error and the intent of the applicant
31.01.03 Insufficient description and specification
31.01.04 Claiming more or less
31.01.06 The application for reissue
31.01.06a Form 1 of Schedule 1
31.01.07 Examination of an application for reissue
31.01.08 Multiple applications for reissue
31.01.08a Examination of multiple, co-existing applications for reissue
31.01.09 Reissue of a reissued patent
31.01.10 Effect of a reissued patent
31.01.11 Appeal from a refusal to grant a reissue
32.02.01 Eligibility Criteria - dates
32.02.01a Examples of calculations of the Later Date
32.02.02 Eligibility Criteria – application by patentee
32.02.03 Dismissal of application due to ineligibility
32.03 Process for determination of additional term
32.03.01 Notice of Preliminary Determination
32.03.03 Certificate or Dismissal
32.03.03a Additional Term Certificate
32.04 Calculation of duration of additional term
32.04.02 Periods of time – general
32.04.03 Periods of time – notices
32.04.03a Notice of non-compliance
32.04.03b Notice of missing application fee
32.04.03d Notice requiring a copy of the priority application
32.04.03e Notice requiring the translation of a priority application
32.04.03f Examiner requisition or final action
32.04.03g Notice requiring a request for continued examination
32.04.03i Conditional notice of allowance
32.04.03j Notice indicating submissions or proposed amendment may be made
32.04.03k Notice of proposed hearing
32.04.03l Notice that amendments must be made
32.04.03m Notice requiring the appointment of a patent agent
32.04.03n Notice of disregarded communication (joint applicant)
32.04.03o Notice of disregarded communication (patent agent)
32.04.03p Notice of disregarded communication (patent agent not named)
32.04.03q Notice to establish entitlement to PCT national phase application
32.04.03r Notice requiring a translation or complete copy
32.04.04 Periods of time – other events
32.04.04a Request for further drawings
32.04.04c Addition of missing parts
32.04.04d Request for further examination or continued examination
32.04.04e Three examiner notices sent
32.04.04g Direction to limit claims
32.04.05 Periods of time - designated days
32.05.01 Initiated by the Commissioner
32.05.02 On application by the patentee or other person
32.05.03 Notice of preliminary determination
32.05.05 Amended certificate or dismissal
Chapter 33 Transitional Provisions
33.02 Communicating with the Patent Office
33.02.01 Presentation of documents
33.02.03 Extension of time to ‘top-up’ small entity fees
33.03 Filing a patent application
33.03.01 Regular Canadian patent application
33.03.02 Divisional applications
33.04.01 Presentation and parts of an application
33.04.02 Statement or declaration of entitlement
33.05.01 Common representative
33.05.02 Appointed patent agents and associate patent agents
33.05.03 Procedures related to patents
33.06.01 Restoration of the right of priority
33.07 Maintenance fees for patent applications
33.08 Abandonment and reinstatement of patent applications
33.09.01 Requesting Examination
33.09.02 Examination in progress
33.10 Allowance, final fee and issuance of patents
33.11 Maintenance fees for patents, deemed expiry and reversal of deemed expiry
33.12 PCT (National Phase Entry)
Chapter 34 Patent Cooperation Treaty – National Phase Entry
34.02 National phase entry requirements for Canada
34.02.01 Priority date used in calculating time limits relating to national phase entry in Canada
34.02.02 Reinstatement of rights for national phase entry
34.02.03 Extension in case of attempted payment
34.02.04 Translation of elements of application
34.02.05 Notice requiring a translation of complete copy
34.02.06 Correction of Error in translations
34.03 National phase entry date
34.04 Open to public inspection (OPI) date for PCT national phase applications
34.05 Notice of discrepancy in applicant name(s)
34.06 Correction of error in the naming of an applicant
34.07 Applicability of Canadian patent legislation
34.09 Filing date of a PCT national phase application
34.10 Restoration of the right of priority
34.11 Form to request national phase entry in Canada
Chapter 35 Guide to Notices, Letters and Requisitions
35.02.01 Information in Commissioner’s notices
35.02.02 List of Commissioner’s notices
35.03.01 Information in courtesy letters
35.04 Examiner requisitions and notices
35.05 Conditional notices of allowance
Date modified: October 2019
This Manual of Patent Office Practice (MOPOP) sets out the administrative and examination practices of the Canadian Intellectual Property Office (CIPO) with respect to patent applications, patents and related procedures. The practices set out in the MOPOP are CIPO's interpretation of the Patent Act, the Patent Rules and jurisprudence as of the date each chapter came into effect.
This manual is a guide only and should not be considered legally binding. If there are inconsistencies between the information in this manual and the applicable legislation, the legislation must be followed. The information provided is for information purposes only and should not be relied upon for legal purposes or business decisions.
We update the manual from time to time to reflect changes to Canada's patent statutes, regulations and jurisprudence.
Please note that the current version of the MOPOP does not cover practices relating to the prosecution of applications filed before October 1, 1989.
You can find information about future updates to this manual, including periods of public consultation, on the MOPOP updates page.
Date modified: June 2021
Through a patent, the government gives you, the patentee, the right to stop others from making, using or selling your invention from the day the patent is granted to a maximum of 20 years after the day on which you filed your patent application. Patents can have a great deal of value. You can sell them, license them or use them as assets to attract funding from investors.
In exchange for these benefits, you must provide a full description of the invention when you file a patent application. This helps enrich technical knowledge worldwide. Details of patent applications filed in Canada are disclosed to the public after an 18-month period of confidentiality.
To be eligible for patent protection, your invention must be:
The invention can be:
In Canada, the first applicant to file a patent application is entitled to obtain the patent. You should file as soon as possible after you complete an invention in case someone else is on a similar track.
People may then read about your invention, though they cannot make, use or sell it without your permission.
The rights given by a Canadian patent extend throughout Canada, but not to other countries. You must apply for patent rights in other countries separately. Likewise, foreign patents do not protect an invention in Canada.
Any public disclosure of an invention before filing may make it impossible to obtain a patent. There is an exception in Canada if the public disclosure was made by the inventor or by someone who learned of the invention from the inventor less than one year before filing the patent application. Similar exceptions apply in some other countries such as the United States. However, please be aware that in some countries disclosing the invention to the public anywhere in the world before filing a patent application may, in many circumstances, prevents the inventor from obtaining a patent.
Most experts agree that inventors should use the services of a licensed patent agent to help with the complexities of patent law. In fact, more than 90 percent of patent applications are filed with an agent’s support.
See A Guide to Patents for information on how to register, key facts, important considerations and more.
Date modified: October 2019
The Canadian Intellectual Property Office (CIPO) is a part of Innovation, Science and Economic Development Canada. CIPO is a Special Operating Agency (SOA) and is responsible for the administration and processing of the greater part of intellectual property in Canada. CIPO's areas of activity include:
CIPO’s mandate is to deliver high quality and timely IP products and services to customers, and to increase awareness, knowledge and effective use of IP by Canadians. Our leadership and expertise in intellectual property support creativity, enhance innovation and contribute to economic success.
Date modified: June 2023
Our Client Service Centre (CSC) provides free support and general information regarding intellectual property, including patents, and the application process. They can be contacted by phone, email or in person.
Note that the CSC does not provide direct action on service requests or payments for any patent or application. The CSC does not undertake the role of an administrative officer, agent, or examiner and, therefore, does not advise on any course of action or give specific guidance with respect to prosecution or any action not strictly prescribed by the Patent Act and Rules.
Upon request, the Patent Office will only provide information concerning the status of a patent application or patent as part of a certified file history, provided that the prescribed fee is paid for each application or patent and the application is open to public inspection. The Patent Document Order Form is available online (see link below).
The Patent Office does not respond to applicant or agent letters (including pending requests) inquiring when a particular application will be examined.
You can order a copy or a certified copy of any specific document or the complete file of a patent application or patent that is open to public inspection. The request can be made at the CSC or online.
Date modified: October 2019
This Chapter provides guidance on the procedures for communicating with the Commissioner of Patents and the Patent Office and is comprised of two sections. The first part, entitled Written Communications, outlines the formalities requirements for paper and electronic correspondence. The second part, entitled Time, explains the legislative provisions for prescribed days, time limits, and extensions of time. Section 12.06 in Chapter 12 details the examination practice for examiner interviews.
Date modified: October 2022
All written communications intended for the Commissioner of Patents or the Patent Office must be addressed to the Commissioner of Patents (section 6 of the Patent Rules). If the communication is written by a patent agent on behalf of an applicant or patentee in respect of a particular patent application or patent, the communication must include the name of the patent agent submitting the communication, unless the agent is taking an action that may be taken by any person authorized by an applicant or patentee (section 41.1 of the Patent Rules). For further information on representation before the Patent Office, please see chapter 5 of this Manual.
A person doing business with the Patent Office via written communication must always provide their postal address (section 7 of the Patent Rules).
Each piece of written communication must, subject to the exceptions set out in Section 2.02.02a of this Chapter, pertain to a single patent application or patent and, at a minimum, must identify the application/patent number and the applicant/patentee name(s) (sections 8 and 9 of the Patent Rules).
Primary communications with the Commissioner must be in English or French in order for the Office to provide a basic level of service. Generally, this requirement extends to any document submitted accompanying or included within the primary communication (section 15 of the Patent Rules), with the exception of the following documents, which may be in a language other than English or French (although a translation will also generally be required):
Date modified: October 2019
All documents submitted to the Patent Office in connection with a patent or a patent application must comply with the form requirements outlined in section 13 of the Patent Rules. The purpose of this is to ensure that the Office can optically scan and digitally store all communications.
Documents submitted in paper form must be:
See the sections on the presentation of application (sections 47 to 52 of the Patent Rules) for the formatting requirements for the description, claims, and abstract.
Refer to Section 2.02.07 of this Chapter for instructions on the formatting requirements of electronic forms of submission.
Date modified: October 2019
Written communications are permitted to relate to more than a single patent application or patent if the communication pertains to the following:
(subsection 8(2) of the Patent Rules)
Date modified: October 2019
If the patent application number is not known (for example in cases where an application number has not yet been assigned), then sufficient information must be given in order to identify that application. The Patent Office will do its best to identify the patent application with the information provided. Please note that the Patent Office databases have limited search functions and we encourage clients to provide as much information as possible when the application number is not known. (subsection 9(1) of the Patent Rules)
Date modified: October 2019
There are specific requirements in the Patent Rules regarding who can communicate with the Patent Office with respect to patent applications and patents for the purposes of certain actions. Chapter 5 contains additional information on Representation.
Date modified: October 2019
The Office strives to maintain current and accurate records. As per section 7 of the Patent Rules, written communications sent to the address, postal or email, provided by the person is considered to have been sent to that person on the date that it bears. It is therefore imperative that persons doing business before the Patent Office update their address in a timely fashion.
Date modified: October 2019
Written communications may be physically delivered to the Patent Office by mail, in person, or to a designated establishment only as outlined in the section below. (Section 10 of the Patent Rules)
For information on the date of receipt accorded to documents, information or fees, please refer to the information contained under the Time heading in section 2.03.
Date modified: December 2020
Written communications addressed to the Commissioner of Patent can be sent by mail or delivered in-person to the Patent Office during ordinary business hours at the following address:
Canadian Intellectual Property Office
Place du Portage I
50 Victoria Street, Room C-114
Gatineau QC K1A 0C9
Please be advised that once communications are received by CIPO they cannot be returned to the sender, even if following its receipt the sender states that the communication was sent in error. When submitting a fee, the Office strongly recommends that the Fee Form be included as a covering document and that it be the only document submitted to CIPO containing financial information, such as credit card numbers.
Date modified: November 2021
For the purposes of subsection 10(1) of the Patent Rules, the Registered MailTM and XpresspostTM services of Canada Post are designated by the Commissioner as being accepted for the physical delivery of documents, information or fees being submitted to the Commissioner or the Patent Office. Written communications addressed to the Commissioner of Patents may thus be sent via the Registered MailTM and XpresspostTM services of Canada Post.
Date modified: October 2019
For the purposes of subsection 8.1(1) of the Patent Act, documents, information or fees may be submitted to the Commissioner or the Patent Office using electronic means only as provided for in this document. Written communications sent online or by facsimile constitutes the original; therefore a duplicate paper copy should not be forwarded. For information on the date of receipt accorded to documents, information or fees, please refer to the information contained under Section 2.03 entitled Time of this Chapter.
Please note that documents, information and fees submitted by electronic means must comply with the electronic format requirements detailed in section 2.02.07 of this Chapter.
Date modified: July 2024
Written communications addressed to the Commissioner of Patents may be sent electronically online using the relevant links below:
Date modified: December 2020
Facsimile correspondence addressed to the Commissioner of Patents may be sent to the following facsimile numbers:
Facsimile correspondence that is sent to any facsimile number other than those indicated above, including those of a designated establishment, will be considered not to have been received.
The electronic transmittal report returned to you following your facsimile transmission will constitute your acknowledgment receipt. Confidentiality of the facsimile transmission process cannot be guaranteed. Please note that CIPO strongly discourages the use of a computer facsimile interface or internet-based facsimile services due to technical issues with reception.
When submitting a document by facsimile that also has a fee requirement, notification of the preferred mode of payment to be applied must be prominently displayed on the Fee Form to ensure expeditious processing.
Date modified: November 2021
Pursuant to PCT Rule 89bis, CIPO, in its role as a receiving Office, accepts the electronic online filing of international applications prepared using WIPO’s ePCT online service. After preparation, filing must be done using CIPO’s International Filing e-service called PCT E-Filing.
Following the initial filing, all documents or correspondence related to a PCT international application may be sent electronically to CIPO via facsimile (see 2.02.06b) or the secure online form using the following link:
Date modified: January 2025
By default, CIPO in its role as a receiving Office, as an International Search Authority and an International Preliminary Examination Authority, will send all PCT international written communications to the postal address provided by the applicant in the request form PCT/RO/101. Under subsection 7(2) of the Patent Rules, the Commissioner has specified Canada Post Connect as means of electronically sending correspondence from the Office. To receive correspondence via Canada Post Connect, applicants must provide their preferred email address of correspondence and a written and signed consent form that is available on CIPO’s website.
Date modified: July 2024
For the purposes of subsection 8.1(1) of the Patent Act, documents, information or fees may be submitted to the Commissioner or the Patent Office on electronic media, such as 3.5 inch diskette, CD-ROM, CD-R, DVD, DVD-R and any format as specified in Annex F of the PCT Administration Instructions.
Documents physically submitted on an electronic medium should include a cover letter and a table of contents, which will be date-stamped by the Office and placed in the application file.
When submitted on electronic media, the parts of the application must be broken down into individual files, each being no larger than 25 megabytes.
The electronic medium must also be free of worms, viruses or other malicious content. Files with malicious content will be deleted.
For information on the date of receipt accorded to documents, information or fees, please refer to the information contained under Section 2.03 of this Chapter, entitled Time.
Date modified: September 2020
For the purposes of subsection 8.1(1) of the Patent Act, documents, information or fees submitted to the Commissioner or the Patent Office online or on electronic media must be in an electronic format provided for in this document.
The Patent Office will accept files in TIFF, PDF, or ASCII formats when they comply with the following specifications:
PDF Format:
ASCII
The Office will accept documents initially filed in other formats provided they are viewable with the software “Stellent Quick View Plus 8.0.0”. In these cases, the Office will request that they be replaced by documents in either PDF or TIFF formats and accompanied by an explanation stating that the replacement documents are identical in content to the documents initially filed.
Date modified: July 2022
When a sequence listing is required under section 58 of the Patent Rules, that sequence listing must be presented in an electronic form and be in compliance with the PCT sequence listing standard.[1]
Date modified: July 2022
Pursuant to PCT Rules 89bis, 89ter and 13ter, and in accordance with Part 7 and Annex C of the PCT Administrative Instructions, where an international application contains disclosure of one or more nucleotide and/or amino acid sequences that are required to be included in a sequence listing, CIPO, in its role as a receiving Office, accepts sequence listings submitted only in electronic form in Annex C/ST.26 compliant XML format:
provided that the other elements of the international application are filed as otherwise provided for under the PCT.
For this purpose the Canadian receiving Office will accept any electronic form of international application specified in Annex F of the PCT Administrative Instructions. A sequence listing which is contained in the international application as filed shall be presented as an electronic file that is part of the application package or on a separate physical medium (which shall contain no other programs or files), even where the remainder of the international application is filed on paper.
For further details concerning the filing of sequence listings and/or tables in electronic form, including the labeling of any electronic media and the calculation of the international filing fee, refer to Part 7 of the PCT Administrative Instructions.
Date modified: June 2021
The Commissioner of Patents or the Patent Office will send written communications to persons doing business before the Patent Office at the postal or email address they provided in accordance with section 7 of the Patent Rules. Unless communications by email is specifically requested and authorized by the person, by default, the Office will send all written communications to the postal address provided.
Note that when the Office is sending a written communication regarding a particular application, patent or other business, and all of the patent agents at a firm are appointed in respect of that business, correspondence sent to the firm is considered to have been sent to all of the agents at the firm (section 29.1 of the Patent Rules). For further information about patent agents and representation before the Patent Office, please see chapter 5 of this Manual.
Date modified: October 2019
It is always the responsibility of the applicant, patentee, or their representative to maintain their address on the patent or patent application file records. In the case of a returned communication, the Office will verify the address on file. If it correct, the Office will resend a courtesy copy to the same address and any consequences resulting from the communication will stand. If the address is incorrect, then the Office will withdraw the communication and reissue it with a new date.
Date modified: December 2020
In the rare case when the applicant, patentee or other person alleges that a written communication from the Office was not received at the postal or email address to which the communication was addressed, the Office will conduct an internal review of its records to ensure that correspondent information originating from a compliant request by the default correspondent has been accurately recorded coincident or prior to the sending of the missing communication. If the Office establishes that the communication was sent to the applicant’s correct recorded address, it will consider withdrawing the communication if an affidavit or statutory declaration containing evidence is submitted in support of the allegation. The Office recommends that the affidavit or statutory declaration contain information about the person’s record keeping systems and copies of the relevant records (e.g. mailroom or email docketing records) to demonstrate that the communication was not received.
If upon review of the affidavit or statutory declaration, the Office is satisfied that the communication was not received at the postal or email address to which the communication was addressed, the Office will withdraw the communication and issue it again with a new date. If the Office is not satisfied, it will inform the person by letter and the Office will consider the communication to have been received.
If upon internal review, the Office determines that an error or delay in its record keeping in relation to the address on file resulted in the missing communication, the Office will withdraw the communication and issue it again with a new due date.
Date modified: September 2020
Occasional but rare technical errors in the Office may result in the inadvertent sending of Notices for actions that are not or no longer prescribed, for example, the sending of Commissioner’s Notices for non-payment of maintenance fee on applications that are already beyond the point of reinstatement or that have been withdrawn. In such instances, the Office will notify the recipient that such notices will be considered never sent and that any such effects produced by those notices will be considered never to have occurred.
Date modified: September 2020
In the cases where the applicant or patentee or other person alleges that a written communication was sent to the Office or the Commissioner and that item appears not to have been received in the Patent Office, the Office will consider an affidavit or statutory declaration submitted with documentary evidence that supports the allegation that an item was received by the Office on a specified day. Documentary evidence may include any indication of reception of a submission or transmission and/ or any indication of payment received by the Office. Re-submission of the documents will also be required.
If upon review of the affidavit/statutory declaration and documentary evidence the Office is satisfied that the communication was received in the Patent Office the communication will be deemed to have been received on the date supported by the documentary evidence. If the Office is not satisfied, it will inform the person by letter and the Office will consider the communication not to have been received.
If the missing communications have resulted in the application becoming abandoned or the patent deemed expired, the applicant or patentee may consider the suitable existing provisions of the Patent Act, be it reinstatement under subsection 73(3) of the Patent Act (see Chapter 9) or reversal of deemed expiry under subsection 46(5) of the Patent Act (see Chapter 27).
Date modified: October 2022
In the rare case when the applicant, or other person alleges that an examiner’s report from the Office was received with a delay of greater than one month from the date on the examiner’s report, at the postal or email address to which the examiner’s report was addressed, the Office will consider withdrawing the examiner’s report if, in addition to the requisition under subsection 86(2) or 86(5) of the Patent Rules, it contains a requisition or requirement under section 85, 85.1 or 94 of the Patent Rules, and an affidavit or statutory declaration containing evidence is submitted in support of the allegation within 14 days of the receipt of the report. The Office requires that the affidavit or statutory declaration contain information about the person’s record keeping systems and copies of the relevant records (e.g. mailroom or email docketing records) to demonstrate that the examiner’s report was received greater than one month from the date on the examiner’s report.
If upon review of the affidavit or statutory declaration, the Office is satisfied that the examiner’s report was received greater than one month from the date of the examiner’s report at the postal or email address to which the examiner’s report was addressed, the Office will withdraw the examiner’s report and issue a new report. If the Office is not satisfied, it will inform the person by letter and the Office will consider the examiner’s report to have been received in a timely manner.
It should be noted that, in certain circumstances, the applicant may instead wish to request an extension of time to respond to the report under subsection 3(1) of the Patent Rules (see sections 2.03.03 and 2.03.03e(ii)) when an examiner’s report is received more than one month from the date of the report. The fee required for an extension of time may be waived in these circumstances if certain requirements are met (see 2.03.03e(ii)).
Date modified: June 2021
Patent agents may have their licence suspended, revoked or surrendered by the College of Patent Agents and Trademark Agents. When this occurs, any appointment of that one particular agent, in respect of any application or patent, is revoked. Note that if all of the patent agents at a firm are appointed, the appointment would be revoked only if the licences of all of the agents at the firm are suspended, revoked or surrendered (paragraph 27(7)(b) and subparagraphs 28(5)(a)(ii), 28(5)(b)(ii), 28(5.1)(a)(ii), and 28(5.1)(b)(ii) of the Patent Rules).
When an appointment of a patent agent is revoked under the above circumstances, any written communication sent to that agent on the day of, or within the four month period preceding the suspension, revocation or surrender of their licence – that has not been responded to – is considered not to have been sent (section 11 of the Patent Rules).
In practical terms, the applicant will be advised of the revocation and any written communications requiring action that were sent in the preceding four months will be reissued with a new due date and sent to the applicant. The applicant may also receive a notice requiring an appointment of agent if an agent is required. For further information about patent agents and representation before the Patent Office, please see chapter 5 of this Manual.
Date modified: October 2019
All documents, information or fees submitted to the Patent Office are accorded a date of receipt in accordance with section 10 of the Patent Rules.
Date modified: October 2019
The date of receipt for physical delivery to the Patent Office depends on whether the Office is open to the public. For a description of the means of physical delivery of documents, information or fees to the Office, see section 2.02.05a. If they are delivered when the Office is open to the public, they are deemed received on that day. If they are delivered when the Office is closed to the public, they are deemed received on the day the Office is next open to the public. (Subsection 10(2) of the Patent Rules)
Date modified: October 2019
The date of receipt for physical delivery to designated establishments depends on whether the Office and the designated establishment are open to the public. For a description of the means of physical delivery of documents, information or fees to designated establishments, see sections 2.02.05b and 2.02.05c.
If they are physically delivered to a designated establishment when it is open to the public and
If they are physically delivered to a designated established when it is closed to the public, then they are deemed received on the first day that the Office is next open to the public that falls on or after the day that the designated establishment is next open to the public. (Subsection 10(3) of the Patent Rules)
Date modified: October 2019
The date of receipt for documents, information or fees submitted by electronic means specified by the Commissioner is the day, according to the local time of the Patent Office, that they are received, regardless of whether the Office is open to the public or not. For a description for submission of documents, information or fees by electronic means, see section 2.02.07.
Date modified: October 2019
There are multiple time limits set in the Patent Act and Patent Rules for submitting documents, information and fees. Time limits are usually expressed as requiring an action within a certain number of months after a specified day.
Date modified: October 2019
When an action is required to be taken within a fixed number of months after a specified day, the time limit is calculated by:
A few examples are listed below for illustrative purposes:
Example 1:
An examiner’s requisition dated January 15 requires a response within four months therefore the time limit for a response is May 15 of the same year.
Example 2:
The maintenance fee for an application is due on Aug 29, 30 or 31 and it is not paid by the due date. The Commissioner’s Notice is sent on September 15 requiring the applicant to pay the fee and late fee before the later of 2 months after the date of the notice or 6 months after the maintenance fee due date. The later date is 6 months from the maintenance fee due date or February 28 (or February 29 in leap years) of the following year.
Example 3:
A Commissioner’s notice sent under section 65 of the Rules requiring the applicant to comply within three months after the notice is sent on March 31. The applicant is required to respond by June 30.
Date modified: October 2019
There are provisions in the Patent Act and Patent Rules which extend time in certain circumstances as described below.
Date modified: October 2022
Under subsection 78(1) of the Patent Act, where a time period for doing anything ends on a prescribed day or on a day designated by the Commissioner, the time period is extended to the next day that is not a prescribed day or a designated day. Section 78 of the Patent Act does not apply to international applications during the international phase (see section 2.03.04).
Date modified: July 2022
The prescribed days for the purposes of subsection 78(1) of the Patent Act are listed in section 5 of the Patent Rules and are copied here for convenience:
a. Saturday
b. Sunday
c. January 1 or, if January 1 falls on a Saturday or a Sunday, the following Monday
d. Good Friday
e. Easter Monday
f. the Monday before May 25
g. June 24 or, if June 24 falls on a Saturday or a Sunday, the following Monday
h. July 1 or, if July 1 falls on a Saturday or a Sunday, the following Monday
i. the first Monday in August*
j. the first Monday in September
j.1 September 30 or, if September 30 falls on a Saturday or a Sunday, the following Monday
k. the second Monday in October
l. November 11 or, if November 11 falls on a Saturday or a Sunday, the following Monday
m. December 25 and 26, or
i. If December 25 falls on a Friday, that Friday and the following Monday, and
ii. If December 25 falls on a Saturday or Sunday, the following Monday and Tuesday
n. Any day on which the Patent Office is closed to the public for all or part of the day during ordinary business hours
*Please note that the Office is open on the first Monday in August.
An example of an extension of time for a prescribed day is provided below for illustrative purposes:
A notice of allowance dated July 11 requires payment of the final fee within four months of the date of the notice (November 11).
Date modified: October 2022
In the case of unforeseen circumstances, the Patent Office will attempt to remain open to the public and ensure that essential service to our clients continues with the least possible disruption or delay. Unexpected closures, whether they are for all or part of the day will be announced on the CIPO website and through social media. These unexpected closures are captured under paragraph 5(n) of the Patent Rules described in section 2.03.03b of this Chapter. For the purposes of subsection 78(1) of the Patent Act, if the time period ends on a day of unexpected closure, the time period is extended to the next day the Patent Office is open to the public. Section 78 of the Patent Act does not apply to international applications during the international phase (see section 2.03.04).
Date modified: October 2022
Under subsection 78(2) of the Patent Act, the Commissioner may designate any day on account of unforeseen circumstances, and if the Commissioner is satisfied that it is in the public interest to do so in order to extend time periods ending on that day. This type of event is described in the business community as a “force majeure” clause, although not described as such in the Patent Act or Patent Rules. This provision allows the Commissioner to suspend obligations when unforeseen circumstances arise making it impossible for applicants and patentees to fulfill them as they would normally be permitted. Any designation of a day or days by the Commissioner will be published on the CIPO website and the time periods that end on the designated day(s) will be extended to the next day the Patent Office is open to the public under subsection 78(1) of the Patent Act. Section 78 of the Patent Act does not apply to international applications during the international phase (see section 2.03.04).
Date modified: January 2025
The Commissioner has the discretionary authority to extend periods of time for certain actions under the Patent Rules if the Commissioner is satisfied that the circumstances justify the extension and the other administrative conditions are met. Under subsection 3(1) of the Patent Rules, applicants and patentees can, prior to the expiry of a time limit, request an extension of time for actions where it is permitted by the Rules (please see Section 2.03.03f of this Chapter for a list of exceptions where extensions of time are not permitted).
The requirements of a compliant request for extension of time under subsection 3(1) of the Patent Rules are shown below, the omission of any one of the following will result in the request being refused by the Commissioner:
The Office will assess the request and if it is compliant and reasonable, the Commissioner will grant an extension of time of up to a period of their discretion. The applicant/patentee will be notified by letter of the Commissioner’s decision. For information on the service standard for this request, please refer to CIPO’s website.
Examples of actions eligible for extension of time include:
If the initial request is refused and if time permits, subsequent requests for an extension of time for the same action on the same file will be considered.
Examples of what could amount to exceptional circumstances that would justify a further extension of time:
Date modified: October 2022
The time to respond to an examiner’s requisition is four months. The Commissioner may extend the time limit to respond to the examiner's requisition to a maximum of six months from the date of the examiner’s requisition under subsection 131(2) of the Patent Rules. Once the Commissioner has made the determination that the extension is justified, the newly established due date will be six months from the date of the examiner’s requisition. The extension of time will not be calculated by the appending of two months from the date of the deemed expiry of the four month due date which may have fallen on a designated or prescribed date.
Any extension of time for an examiner’s requisition will not apply to any requirement to request continued examination that might form part of an examiner’s report.
Date modified: October 2022
The time to respond to an examiner’s requisition is four months. In the case where the examiner’s requisition under 86(2) or 86(5) of the Patent Rules was received more than one month after the day on which it was sent, the Commissioner may extend the time limit to respond to the examiner’s requisition to a maximum of six months from the date of receipt of the examiner’s requisition if the applicant:
Evidence satisfactory to the Commissioner may include official reports of incoming correspondence identifying the examiner’s requisition or with the associated date of receipt, a copy of the correspondence with a date-stamp, or other data or reports that clearly show the date of receipt of the examiner’s requisition.
If this information is provided by the applicant, the Commissioner will waive payment of the prescribed fee to apply for an extension of time (see section 10.05).
If the Commissioner is satisfied that the circumstances justify the extension, the newly established due date will be four months from the day on which the examiner’s requisition was received. Requests for an extension of time beyond 4 months from the established date of receipt will be considered based on the circumstances and the justifications submitted. The fee for requesting an extension of time beyond 4 months from the date of receipt will not be waived.
Date modified: October 2022
While extension of time may be granted for certain actions, there are time limits that are fixed under the Patent Rules for which subsection 3(1) of the Patent Rules does not apply in those circumstances. The following is a list of actions for which the Commissioner cannot grant an extension of time under the Patent Rules:
Please note that time limits that are fixed under the Patent Act cannot be extended under subsection 3(1) of the Patent Rules. The period of time fixed by subsection 18(2) of the Patent Act is however extendable under section 4 of the Patent Rules.
Date modified: January 2025
The Commissioner is authorized to extend the period of time for the payment of fees paid at the small entity rate after the expiry of that period if the Commissioner considers that the circumstances justify the extension and if the following requirements are met:
The applicant will be notified by letter of the Commissioner’s decision regarding any request for an extension of time. The following fees are eligible for a ‘top-up’:
Please note that the Patent Office will accept a single request and statement (under paragraph 3(3)(c) of the Patent Rules) to cover the ‘top-up’ of multiple fees at the small entity rate so long as the applicant/patentee pays the extension of time fee for each fee previously paid at the small entity rate that is being “topped-up” as well as the difference for each fee.
Where the Patent Office determines that the request is compliant, the ‘top-up’ payments are considered to have been made on the date of the original small entity fee payments (subsection 3(5) of the Patent Rules). If part of the compliant request includes a ‘top-up’ payment for a fee that does not require an extension of time, as the deadline for that fee has not yet passed, this payment is also considered to have been made on the date of the original small entity payment (section 5.01 of the Patent Rules). Following a successful request to ‘top-up’ all small entity fees, the Patent Office will update the entity size in its records and all future fees and corresponding notices and letters will list the fee at the standard rate.
The Patent Rules do not allow for the topping up of an application fee paid prior to October 30, 2019. Therefore, any top up request for such a fee will not be accepted and the application will continue to show future fees at the small entity rate.
If not all fees have been “topped-up”, the entity size in the Patent Office records will remain small for the purposes of determining fees, corresponding notices and letters. Applicants and patentees may still pay any future fees at the standard rate though the difference will remain on file at the Patent Office and be available for refund within three years of payment upon request.
Date modified: January 2025
The Commissioner is authorized to extend the period of time for the payment of a fee for examination of an application (see Patent Rules, subsection 80(1)). The Commissioner can, under subsection 3(3.1) of the Patent Rules, allow the extension to be made after the end of the time limit to request examination (see 11.01.02) if the Commissioner considers that the circumstances justify the extension and if all of the following apply:
The applicant will be notified by letter of the Commissioner’s decision regarding any request for an extension of time.
Where the Patent Office determines that the request is compliant, the ‘top-up’ payment is considered to have been made on the date of the original insufficient request for examination payment (subsection 3(5) of the Patent Rules).
Date modified: January 2025
The Commissioner is authorized to extend the period of time for the payment of a fee after the expiry of that period if the Commissioner considers that the circumstances justify the extension and if the following requirements are met:
If the requirements are met, the ‘top-up’ payment will be considered to have been made on the date of the original insufficient payment (subsection 3(5) of the Patent Rules). Where applicable, the Office will reverse any consequence that may have occurred due to the insufficient fee being paid before the expiry of the period to pay the fee.
Date modified: October 2022
Section 78 of the Patent Act does not apply to international applications during the international phase. For information about extensions of time during the international phase see MOPOP Section 34.08.
In accordance with section 160 of the Patent Rules, section 78 of the Patent Act does not apply to PCT national phase applications in respect of a time period that ended before the national phase entry date.
Date modified: October 2019
Obtaining a patent in Canada starts by submitting and prosecuting a patent application. There are three types of patent applications:
This Chapter details the documents and information that must be submitted in order to secure a filing date for a regular filed patent application, request national phase entry for a PCT application and file a divisional application.
Date modified: October 2019
A patent application filed in Canada under the Patent Act is known as a regular Canadian patent application. This distinction from other types of patent applications (PCT National Applications and Divisional Applications) is made only in this Chapter.
Date modified: October 2019
In order to secure a filing date under subsection 28(1) of the Patent Act, an applicant is required to provide the following documents and information, as prescribed by section 71 of the Patent Rules:
When the prescribed documents and information are submitted on different dates, the filing date accorded to the patent application will be the latest of those dates.
Submitting an application fee is not a requirement to secure a filing date for your patent application. If the application fee is not submitted when the patent application is filed, the Commissioner will send the applicant a notice, as required by subsection 27(7) of the Patent Act, requiring the submission of the application fee and the late fee within three months of the date of the notice. If the application fee and late fee are not submitted within the three months after the date of the notice, the application will be considered withdrawn pursuant to subsection 66(2) of the Patent Rules.
Date modified: October 2019
If any of the required documents and/or information is not contained in the application, the applicant will be notified, as required by subsection 28(2) of the Patent Act, of any missing document(s) or information. The applicant will be required to submit the outstanding documents or information within two months after the date of the notice.
If the applicant submits the outstanding documents or information within two months after the date of the notice, the filing date accorded to the patent application will be date on which the last document or information required to establish the filing date was submitted.
If the applicant does not submit the missing documents and information within the two-month period after the date of the notice, the application will be deemed to never have been filed pursuant to subsection 28(3) of the Patent Act.
Date modified: October 2022
The document describing the invention (the description) does not need to be in English or French to establish a filing date. However, if the filing date is established using a foreign language description, the applicant is required to submit an English or French translation of any part of the specification or the drawings that, on the filing date, was not entirely in English or French. If the required translation is not submitted, the Commissioner will send the applicant a notice, as required under subsection 15(4) of the Patent Rules, requiring the submission of the translated document to be submitted not later than two months after the date of the notice
If the applicant submits the English or French translation within two months after the date of the notice the translation will replace the original document. If the applicant does not submit the English or French translation within the prescribed time, the application will be deemed to be abandoned under subsection 73(2) of the Patent Act, as prescribed by subsection 132(1)(a) of the Patent Rules. For more information on abandonment and reinstatement of patent applications, please consult Chapter 9.
Date modified: January 2024
Subsection 27.01 of the Patent Act permits an applicant to submit a reference statement to the Commissioner instead of the description, in order to secure a filing date. The reference statement must be in English or French and to the effect that a reference to a specified previously filed application for a patent is being submitted instead of all or part of the specification or drawing that is required to be contained in the application.
The prescribed period to make a complete reference statement begins the date on which we receive any document to establish a filing date and ends the earlier of:
a)two months from receipt of the earliest document or information required for establishing a filing date or, if a notice is sent under subsection 28(2) Patent Act, the earlier of:
two months after the date of the notice; and
six months after receipt of the earliest document or information required for establishing a filing date; and
b)the filing date.
A reference statement can’t be made to secure a filing date for a divisional application.
Date modified: October 2019
The reference statement must include the following information, detailed in paragraph 67(2)(a) of the Patent Rules, regarding the previously filed application:
Date modified: October 2019
The applicant has two months from the date the reference statement was submitted to the Commissioner to submit a copy of the previously filed application to the Commissioner as outlined in paragraph 67(2)(b) of the Patent Rules. The applicant can either submit a copy or make it available to the Commissioner in a specified digital library and inform the Commissioner that the copy is available. If the previously filed application was filed in Canada, the applicant is exempted from the requirement to provide a copy.
If the applicant meets all the requirements, the specification or drawings in the previously filed application are deemed by subsection 27.01(2) of the Patent Act to have been contained in the application on the date on which the statement is received and this will be the date of submission for the description of the patent application. The filing date of the application will be the latest of the dates when the documents and information required under section 71 of the Patent Rules are submitted.
Date modified: October 2022
If all or part of the previously filed application is in a language other than English or French, the applicant must provide a translation into English or French as outlined in subsection 15(2) of the Patent Rules. If the applicant does not submit the translation when they submit the copy of the previously filed application, the Commissioner will send the applicant a notice, pursuant to subsection 15(4) of the Patent Rules, requiring the submission of the translated document within two months of the date of the notice.
If the applicant submits the English or French translation within two months after the date of the notice, the translation will replace the original document. If the applicant does not submit the English or French translation within the prescribed time, the application will be deemed to be abandoned under subsection 73(2) of the Patent Act, as prescribed by subsection 132(1)(a) of the Patent Rules. For more information on abandonment and reinstatement of patent applications, please consult Chapter 9.
Date modified: October 2019
In the rare circumstance that an applicant mistakenly files the wrong specification or drawings, or they neglect to include part of the specification or drawing, it may be possible to make an addition under section 28.01 of the Patent Act. There is a narrow window of time to make an addition and it may affect the filing date. For that reason, applicants are encouraged to ensure that the documents they submit to establish a filing date are complete and error free.
If an application is missing a part of the specification or a drawing referred to in the application, the applicant may add the missing part to their application by submitting the addition along with a statement indicating that the addition is made under section 28.01 of the Patent Act.
If within two months after the earliest date on which the Commissioner receives any document or information under subsection 28(1) of the Patent Act, the Commissioner finds that part of the description or a drawing appears to be missing, they will notify the applicant of the missing element by a notice under subsection 72(1) of the Patent Rules. Please be advised that the Office will generally verify page numbering continuity and will generally verify that the submission for a filing date contains all listed items. For that reason, applicants are encouraged to submit a summary list of the parts of their application in a submission cover page so that the Office may reconcile it (e.g. abstract 1 page, description: 6 pages, claims: 20 in 4 pages, drawings: 7 in 5 pages).
Date modified: October 2019
Applicants have two months from the earliest date on which the Commissioner receives any document or information required for establishing a filing date, pursuant to subsection 28(1) of the Patent Act, to add the missing part to the application.
If the Commissioner notifies the applicant of a missing part, the applicant must make the addition before the earlier of:
Date modified: October 2019
If the missing part and drawing are completely contained in a prior application on which priority has been requested, the applicant may submit the missing information without affecting the filing date.
In order to secure the original filing date, the applicant will have to ensure the following, under subsection 28.01(2) of the Patent Act:
If the prior application was not filed in Canada the applicant is required to either provide a copy of the prior application or make a copy available to the Commissioner in a digital library that is specified by the Commissioner as being acceptable.
If any part of that prior application is in a language other than English or French, the applicant is required to provide a translation in English or French of that part. They must also indicate where in that prior application or in the translation the addition is contained.
Where the parts being added are not contained in a prior application on which priority has been requested, and the request is not withdrawn before the prescribed date, the missing parts will be added to the application and the filing date will be the later of the date on which the addition is received and the filing date (where other filing requirements have not been met before the addition of the missing part is requested). The consequence is an amended filing certificate.
Considering the short timelines, the Patent Office will aim to expedite the assessment of whether or not the parts being added are contained in the priority application. The Patent Office will do its best to inform applicants rapidly if they are not to give the applicant the opportunity to withdraw the addition and maintain the original filing date.
Since the potential consequence of an addition to the specification may result in a later filing date, applicants should ensure their original submission of their patent application is complete and free of errors.
Addition of missing parts does not apply to divisional applications.
Date modified: October 2019
The application fee is not required to establish a filing date. If an applicant does not pay the application fee on the filing date of the application, the Commissioner will notify the applicant that the application fee and the late payment fee are required to be paid within three months of the date of the notice (subsection 27(7) of the Patent Act, subsection 66(1) of the Patent Rules). If the applicant does not pay the application fee and the late payment fee within the three month period after the date of the notice, their application will be considered to have been withdrawn (subsection 66(2) of the Patent Rules).
An applicant who meets the small entity status condition and submits the small entity status declaration can pay a reduced fee. For more information on small entity fees, please see Chapter 10.
Date modified: October 2019
An application for a patent is given a unique number once a filing date has been established. Patent applications and any resulting patent bear the same number. Applications have been numbered sequentially in Canada since the first Patent Act in 1869, and by the late 1980s, applications reached the one million series. In order to distinguish patent applications filed following amendments to the Act which came into force on October 1, 1989, the numbering was skipped ahead to the two million series, starting with 2 000 000 on that date. As over 1 000 000 applications have been filed since October 1, 1989, a patent application filed today will be numbered in the three million series. A reissued patent and a re-examined patent will bear the same number as the original patent. Divisional applications are also given a number in the series but different from that of the original patent application.
Date modified: October 2019
Once the applicant has established a filing date by submitting the required documents and information to the Office, the Commissioner will send a filing certificate with a unique application number to the default correspondent on file. For information on default correspondent please see Chapter 5.
Date modified: October 2022
An application may be withdrawn at any time. A request for withdrawal must be made in writing from the person authorized to represent the applicant(s) (for more information on representation, see Chapter 5). The application fee referred to in subsection 27(2) of the Patent Act is not refundable. Other fees which have been paid prior to the date of withdrawal may be refunded under paragraph 139(1)(b) of the Patent Rules.
Date modified: October 2019
An international application filed under the Patent Cooperation Treaty may enter the national phase in Canada upon meeting the requirements outlined in section 154 of the Patent Rules. Once the application has entered the PCT national phase in Canada, it is considered an application for a patent filed in Canada starting on its national entry date. The international filing date becomes its filing date in Canada and the prosecution of the patent application will continue under the Patent Act and Rules.
For more information on the Patent Cooperation Treaty, please see Chapter 34.
Date modified: October 2019
A divisional application is a separate patent application which is divided from an original application where the original patent application describes more than one invention. The divisional application benefits from the same filing date as the original application.
The following sections describe the administrative requirements regarding the filing of a divisional application.
Date modified: October 2019
In accordance with subsection 36(4) of the Patent Act, a divisional application is deemed to be a separate and distinct application under the Act, to which the Act's provisions apply as fully as may be, and separate fees shall be paid on the divisional application and, except for the purposes of subsections 27(6) and (7) of the Patent Act, it shall have the same filing date as the original application.
The Patent Office takes the position that a divisional application may itself be considered an original application under section 36 of the Patent Act for the purposes of the filing of further divisional applications.
Thus, if a first application (the "grandparent" application) leads to a first divisional application (the "parent" application), a further divisional application (the "child" application) may be filed on the basis of either the parent or the grandparent application.
Date modified: October 2019
In order to file a divisional application, the applicant must meet the majority of the requirements in section 89 of the Patent Rules on the presentation date set out in section 103 of the Patent Rules and as described in the paragraph below.
The requirements to establish a presentation date are similar to the requirements to establish a filing date of a regular application:
Unlike a regularly filed application, the description for a divisional application, or the document that on its face appears to be a description, subsection 15(1) of the Patent Rules requires the description to be in English or French. An application for a divisional application must also meet the requirements set out in section 89 of the Patent Rules. These requirements are that:
Date modified: October 2019
An applicant may provide the original application number after the presentation date; however, the Office will not be able to process the application unless the original application number is provided. Therefore, it is recommended that that the original application number is provided on the presentation date.
If the applicant provides an original application number on the presentation date and later discovers that the incorrect original application number was provided, the applicant can submit the correct number to the Office not later than three months after the presentation date. Office records will be updated accordingly.
Date modified: October 2022
The Office recommends that applicants include all subject matter relevant to the invention permitted under section 91 or 155.7 of the Patent Rules on the presentation date since all future amendments to the specification and drawings of the divisional application will be assessed on the basis of the subject matter submitted at that time.
For more information on new subject matter and how it is evaluated during examination, please see Chapter 20.
Date modified: October 2019
Each divisional application requires the applicant to pay the application fee though the fee is not required to establish the filing of a divisional application. If the application fee is not submitted on the presentation date, the Commissioner will send a notice under subsection 27(7) of the Patent Act requiring the applicant to pay the fee and the late fee before the end of the three month period after the date of the notice.
Date modified: October 2019
Applicants must pay maintenance fees for divisional applications, separate and distinct from those of the original application. Maintenance fees will be calculated from the filing date of the original application and are payable at the presentation date of the divisional application. (Subsection 68(2) of the Patent Rules)
For example, if a divisional application is filed 40 months after the filing date of the original application, maintenance fees for the 2nd and 3rd year anniversary of the filing date have to be paid at the presentation date upon filing of the divisional application. If the required maintenance fees are not paid at the presentation date, the Commissioner will send a notice under paragraph 27.1(2)(b) of the Patent Act requiring the applicant to pay the maintenance fees and the late fee before the later of two months from the date of the notice or six months from the presentation date. A single late fee will apply.
Date modified: October 2019
Except for the requirement to provide the original application number, the applicant must meet the requirements in section 89 of the Patent Rules on the presentation date in order for the application to be a divisional application. The Patent Rules do not allow for an application to be a divisional application if the requirements in section 89, except for the requirement to provide the original application number, are complied with after the presentation date.
Therefore, if an applicant meets the filing date requirements as described in section 3.04.02 above, but does not meet the requirements for an application to be a divisional application on its presentation date (except for the requirement to provide the original application number), the presentation date will become the filing date of the application. In other words, the application will be treated as a regularly filed application. If this is the case, the Patent Office will send the applicant a filing certificate for a regular application rather than a divisional application.
If the applicant does not successfully file a divisional application, the applicant may wish to withdraw the regular application and re-file the application as a divisional by meeting the presentation date requirements as well as the requirements under section 89 of the Patent Rules, except for the requirement to provide the original application number, on the same date.
Date modified: October 2019
A divisional application cannot be filed if the original application is granted, or if it is abandoned beyond the period of reinstatement. A divisional application may be filed after the original application is refused, if it is filed within the time prescribed in section 90 of the Patent Rules.
An attempt to file a divisional application after the deadline to file a divisional has passed, that meets the requirements to obtain a filing date, will be treated as a regular application and a filing certificate will be sent to the applicant.
Date modified: October 2019
Under section 92 of the Patent Rules, various actions taken in respect of the original application are deemed to have been taken in respect of the divisional application if the action was taken before the presentation date of the divisional application.
These actions are:
The Patent Office will ensure that records for the divisional application reflect the actions that were taken in respect of the original application.
Date modified: October 2019
If the applicant does not comply with a notice that was sent with respect to the original application before the presentation date of the divisional application, the notice will continue to apply to the original application. A divisional application will be assessed for compliance with requirements in the Patent Act and Patent Rules and will be subject to separate notices, if applicable.
Date modified: October 2019
Where a divisional application is filed after the expiry of the eighteen-month confidentiality period specified in section 10 of the Patent Act of the original application, the application and any documents filed in connection with it shall be open to public inspection immediately upon filing. Note that the confidentiality period of a divisional application is calculated based on the earliest filing date of any previously filed application on which a request for priority is made in respect of the divisional application.
Date modified: September 2020
A patent application consists of many parts, only a few of which are required to be submitted to the Patent Office to obtain a filing date. However, all the parts of the patent application must be submitted in order to be compliant with the prescribed requirements of the Patent Act and the Patent Rules. There are also requirements for each of those parts that are assessed for compliance after the filing of a patent application and during its prosecution until either a patent is issued or an application is refused.
This chapter relates to the assessment of patent application requirements specific to submitting parts of the application or statements relating to entitlement to the application, as well as to how and when they are assessed. Other assessments made on the filing date of the application that are not covered in this chapter include payment of the application fee (see section 3.02.06) and appointment of agent (see section 5.05.01),
Requirements related to patentability, such as novelty, obviousness and utility, are assessed by examiners once a request for examination has been made. Assessment of these other requirements is described in more detail in Chapters 12-23 of this manual.
A compliant patent application must contain:
Date modified: October 2019
Information or notices included with an international application, as filed, or information or notices furnished in accordance with the PCT before the application becomes a PCT national phase application, are deemed to have been received by the Commissioner on the international filing date, or on the day on which they were furnished, respectively. Therefore, on the national phase entry date, many PCT national phase applications are already compliant with the administrative requirements of the Patent Act and the Patent Rules.
Date modified: October 2022
After a patent application has received a filing date, or has entered the national phase via the PCT, the Patent Office will review the application to determine if all the required parts have been submitted. If a required part, other than a sequence listing or drawings, has not been submitted, the Commissioner will send a notice under section 65 of the Patent Rules requiring the applicant to comply with the prescribed requirements. The applicant will have three months to respond to the notice in good faith in order to avoid their patent application being deemed to be abandoned under subsection 73(2) of the Patent Act, as prescribed under subsection 132(1)(d) of the Patent Rules.
After all required parts of the application are submitted, certain parts of the application (i.e. the specification, claims, abstract and drawings) will not be thoroughly assessed for compliance until the application is examined by a patent examiner. If these parts of the application are non-compliant, defects may be identified in an examiner’s report.
PCT national phase applications will also be assessed to ensure that translation requirements are met on the national phase entry date. The Commissioner may send a notice under subsection 155.5(6) of the Patent Rules if certain elements or parts of the application are not translated (see section 34.02.05 for more information).
Date modified: October 2022
The Patent Office will review the applicant’s response to a notice sent under section 65 of the Patent Rules and assess whether it renders the application compliant. If the application remains non-compliant following the response, a new Commissioner's notice of non-compliance under section 65 of the Patent Rules will be sent to the applicant. The applicant will once again have three months to respond to the notice in good faith in order to avoid their patent application being deemed abandoned under subsection 73(2) of the Patent Act (subsection 132(1)(d) of the Patent Rules).
The Patent Office will review the applicant’s response to a notice sent under subsection 155.5(6) of the Patent Rules. If the applicant does not comply with the notice, the application will be deemed to be abandoned under subsection 132(1)(h) of the Patent Rules (see section 34.02.05).
Date modified: September 2020
A patent application, except for a PCT national phase application, must contain a petition that complies with section 53 of the Patent Rules. Aside from the requirements set out in section 53 of the Patent Rules, an applicant may wish to include other information or regulatory requirements in the petition, such as a small entity declaration, a request for priority, the statement and information required under section 54 of the Patent Rules to establish entitlement to apply for a patent, and any necessary appointments of a common representative, patent agent, or associate patent agent.
Applicants may use the form here[2] or use their own petition form.
Date modified: October 2022
Pursuant to subsection 54(1) of the Patent Rules, the application must indicate the name and postal address of each inventor of the subject-matter of the invention for which an exclusive privilege or property is claimed.
The application must also contain:
The statements or declaration referred to above must be included in the petition or submitted in a document other than the abstract, specification or drawings. For PCT national phase applications, if all or part of a declaration submitted under 4.17(ii) of the Regulations under the PCT is in a language other than English or French, the applicant must submit an English or French translation to the Commissioner.
If the inventor information or statement referring to the applicant’s entitlement to the application is not provided on the filing date, the Commissioner will send a notice under section 65 of the Patent Rules requiring the applicant to provide the missing information or statement not later than three months after the date of the notice.
Date modified: September 2020
If it appears that drawings were intended to be included in an application at its filing date, but the drawings seem to be is missing, the applicant will be notified by a Commissioner’s notice under subsection 72(1) of the Patent Rules if the application is not a PCT national phase or divisional application. If the application is a PCT national phase application or a divisional application, a notice under subsection 72(1) of the Patent Rules is not applicable, however, a courtesy letter will be sent to the applicant to let them know that the drawings appear to be missing.
If, during examination, the examiner determines that drawings are required to be submitted for the patent application to be compliant, the examiner will identify this requirement in their report. See chapter 12.05.01 for more information.
Date modified: July 2022
If it appears that a sequence listing was intended to be included in an application on its filing date, but it seems to be is missing, the applicant will be notified by a Commissioner’s notice under subsection 72(1) of the Patent Rules if the application is not a PCT national phase or divisional application. If the application is a PCT national phase application or a divisional application, a notice under subsection 72(1) of the Patent Rules is not applicable, however, a courtesy letter will be sent to the applicant to let them know that the sequence listing appears to be missing.
A sequence listing that is provided at filing or national phase entry, or any other time during the application phase, will be reviewed to ensure that it is compliant with the PCT sequence listing standard. If the sequence listing is non-compliant, the applicant will be notified by a Commissioner’s notice under section 65 of the Patent Rules, or by requisition of the examiner, requiring the applicant to comply with the standard. If an application originally filed without a sequence listing is amended to include one, in accordance with subsection 58(3) of the Patent Rules, the applicant must file a statement that the listing does not go beyond the disclosure in the application as filed.
If, during examination, it is determined that a sequence listing is required to be submitted for the patent application to be compliant, the examiner will identify this requirement in their report. See chapter 23.05.07a for more information.
Date modified: October 2019
If an applicant submits a document that appears to be a description for the purpose of obtaining a filing date or a copy of a previously filed application under paragraph 67(2)(b) of the Patent Rules and that document is partly or entirely in a language other than English or French, the applicant must submit to the Commissioner a translation of the document or previously filed application in English or French.
Date modified: September 2020
If the applicant does not provide the required translation on the filing date, the Commissioner will send a notice under subsection 15(4) of the Patent Rules requiring the applicant to submit the translation not later than two months from the date of the notice.
Date modified: October 2019
The description, claims, abstract and drawings must be presented in a way that allows the Patent Office to easily process, scan and read the parts of the application. Sections 47 to 52 of the Patent Rules set out requirements relating to page margins, line numbering, line spacing, font size, page numbering and identification of trademarks. The Patent Rules require that each part of the application begin on a new page, and that other requirements relating to the inclusion of drawings and formulas be met. If the parts of the application are non-compliant with those requirements, the Office may identify a defect in an Office letter or in an examiner’s report. See chapter 2.02.02 for more information.
Date modified: June 2021
Patent agents are individuals who hold a patent agent licence or a patent agent in training licence issued by the College of Patent Agents and Trademark Agents. This licence allows patent agents to represent applicants, patentees and other clients before the Patent Office. Most applicants choose a patent agent to prosecute their patent application on their behalf. The Office recommends that all persons submitting a patent application consult a licensed patent agent for advice, regardless of whether or not the Patent Rules require them to do so.
This Chapter describes provisions in the Patent Rules with respect to the Register of Patent Agents, representation of applicants and patentees by common representatives and patent agents, and who is permitted to take various actions on behalf of those applicants and patentees.
Date modified: June 2021
The Register of Patent Agents is managed by the College of Patent Agents and Trademark Agents (College), in accordance with the College of Patent Agents and Trademark Agents Act and the College of Patent Agents and Trademark Agents Regulations. Requirements to obtain and maintain a patent agent licence are set by the College. The College may also choose to suspend, revoke or surrender the licence of a patent agent. This would result in the revocation of that agent in respect of any appointments before the Patent Office (see chapters 5.04.06 and 5.05.03 of this Manual for more information about revocations of appointments of patent agents).
The Patent Office relies on the College to supply it with updated information about new entries on the Register of Patent Agents, as well as any changes to the status of any agent’s existing licence.
Date modified: June 2021
While the College of Patent Agents and Trademark Agents provides the Patent Office with information about patent agent licences, the Office does not rely on the College to obtain agents’ contact information. As such, if a patent agent moves or changes firms, they should immediately notify the Patent Office of their new address, in order to avoid any potential Office errors.
Changes to the preferred method of correspondence with respect to any particular patent application or patent must also be communicated to the Office.
Date modified: June 2021
In addition to regulating patent agents, the College of Patent Agents and Trademark Agents maintains a list of registered foreign practitioners. These practitioners are persons who are recognized as patent agents under the laws of a country other than Canada, and have limited ability to act on behalf of patent applicants, patentees and other persons under Canada’s Patent Rules. Please note that because they are not granted a full licence by the College, these registered foreign practitioners are not considered ‘patent agents’ for the purpose of the Patent Rules or this Manual, and cannot be appointed in respect of an application, patent or any other business before the Office.
To act on behalf of an applicant or patentee, a registered foreign practitioner must:
Updates to the list of registered foreign practitioners will be provided by the College to the Patent Office in a similar manner as any information pertaining to patent agents’ licences. For information about which specific actions may be taken by registered foreign practitioners, please refer to chapters 5.05 and 5.07 of this Manual.
Date modified: October 2019
The common representative is a joint applicant or joint patentee who is entitled to act on behalf of the other joint applicants or joint patentees in some actions before the Office, in particular the appointment of patent agents. In all cases where there are joint applicants or joint patentees, one of those joint applicants or joint patentees will be the common representative. The joint applicants or patentees may collectively appoint one person as the common representative. Otherwise, one of the joint applicants or patentees will be deemed to be appointed as the common representative by default, in accordance with the Patent Rules.
Date modified: October 2019
For non-PCT applications, the common representative may be appointed in the petition of the application, if that petition is included in the application on the filing date (paragraph 26(3)(b) of the Patent Rules). For PCT national phase applications, the common representative may be appointed in the request for national phase entry or in a notice submitted to the Commissioner at the PCT national phase entry date (paragraph 26(3)(c) of the Patent Rules). These appointments do not require a signature.
Date modified: September 2020
The common representative may also be appointed at any time during the life of an application or a patent in a notice to the Commissioner signed by each of the other joint applicants or joint patentees (paragraph 26(3)(a) of the Patent Rules). Any previous appointment of a common representative is revoked by the appointment of a new common representative (subsection 26(12) of the Patent Rules).
The Office recommends that joint applicants appoint their common representative in the petition or request for national phase entry.
Date modified: October 2022
If no common representative is appointed by the other joint applicants, one of the joint applicants will be deemed to be appointed as common representative, as follows:
Exception (subsection 26(6) of the Patent Rules): where there has been a default appointment of the common representative (rather than an appointment by the other applicants under subsection 26(3) of the Patent Rules), the common representative may change in the following circumstances:
Where there has been a correction to the naming of a joint applicant under section 104 or subsection 154(6) of the Patent Rules which has changed the identity of that joint applicant, or a decision under section 31 of the Patent Act to add or remove an applicant to the application, the joint applicant whose name appears first in alphabetical order is deemed to be appointed as the common representative. If there have been more than one such correction(s) or decision(s), the joint applicant whose name appears first when listed in alphabetical order after the most recent correction or decision is deemed to be appointed as the common representative (subsection 26(6) of the Patent Rules).
If as a result of a correction or decision described above, the existing common representative is no longer a joint applicant, that person will no longer be the common representative, whether they had initially been appointed by the other joint applicants or by default. In this case, as above, the joint applicant whose name appears first in alphabetical order is deemed to be appointed as the common representative. For example, if the Commissioner made a decision under section 31 of the Patent Act that the common representative was no longer allowed to proceed with the application, that person could no longer be the common representative, even if they had previously been appointed as such by the other applicants under subsection 26(3) of the Patent Rules. The new common representative would be the joint applicant whose name appears first in alphabetical order.
The following flow charts illustrate the appointment of a common representative for Regular Canadian (non-PCT) applications, PCT applications and divisional applications, and how the common representative may change due to a correction or decision referred to in subsection 26(6) of the Patent Rules. For an explanation of how a transfer of rights to an application or patent may result in the appointment of a common representative by default, please see the text and flow charts in Section 5.03.05 of this chapter.
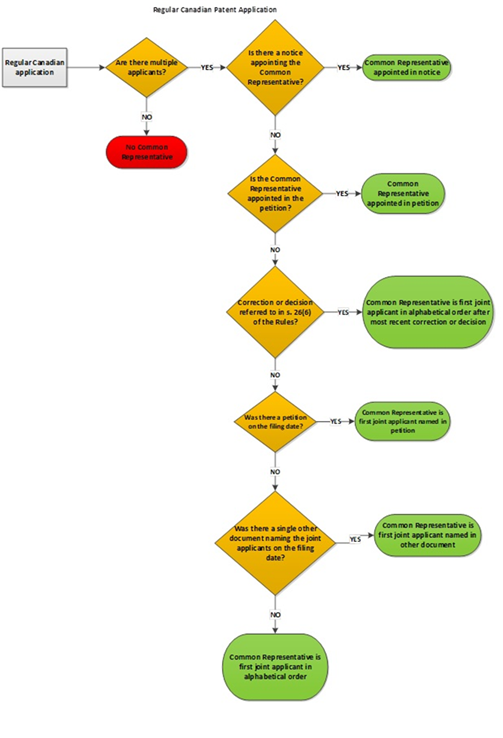
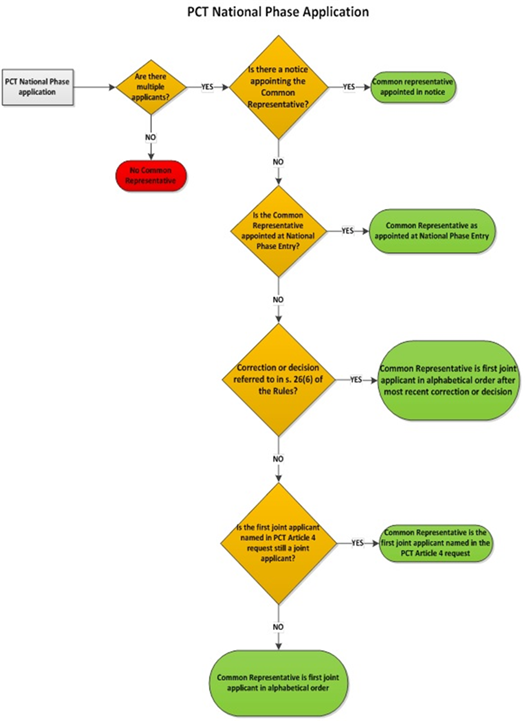
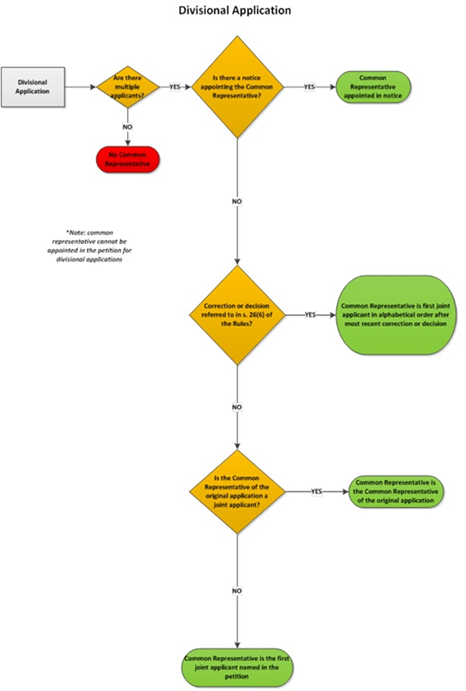
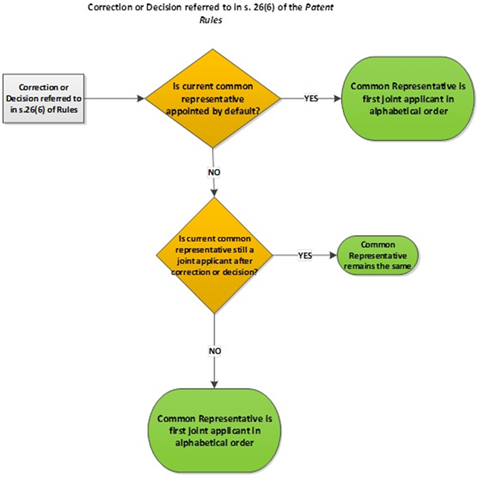
Date modified: October 2019
In accordance with subsection 26(7) of the Patent Rules, the person who was the common representative immediately before the patent was granted will remain the common representative once the patent is granted unless the joint patentees make a new appointment under paragraph 26(3)(a) of the Patent Rules. The same is true for reissued patents (subsection 26(8) of the Patent Rules).
Date modified: September 2020
This section pertains to situations where a transfer occurs and no new appointment of common representative is submitted with the transfer request. The recording of a transfer of rights to an application or a patent under section 49 of the Patent Act will affect the appointment of common representative (where deemed or not) if the full rights of the common representative are the subject of that transfer. So long as the appointed or deemed appointed common representative remains a joint applicant or patentee, the common representative will remain the same.
Where the full rights to a patent application or patent of the common representative are transferred and recorded under section 49 of the Patent Act to one person, that person, the transferee, is deemed to be appointed as common representative in respect of the application or patent. If the common representative transfers all of their rights to multiple persons, or transferees, the transferee whose name appears first in the request to record the transfer is deemed to be appointed as the common representative (subsection 26(11) of the Patent Rules).
When the rights of a single applicant or patentee are transferred, and after the transfer there is more than one applicant or patentee, there must be a common representative. If the person who was the single applicant or patentee prior to the transfer is a joint applicant or joint patentee after the transfer, they are deemed to be appointed as the common representative. In any other case, the transferee whose name appears first in the request to record the transfer is deemed to be appointed as the common representative (subsection 26(9) of the Patent Rules).
The following flow charts illustrate the determination of the common representative in the case of a transfer.
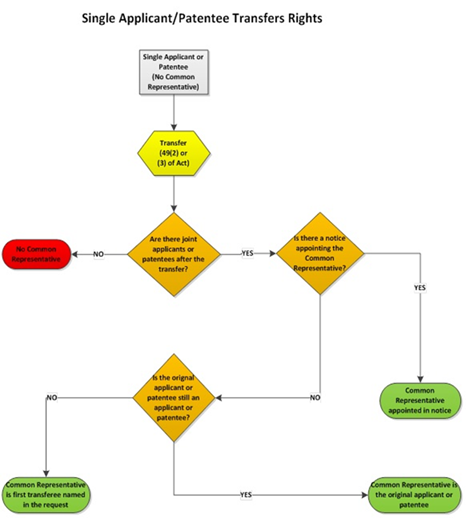
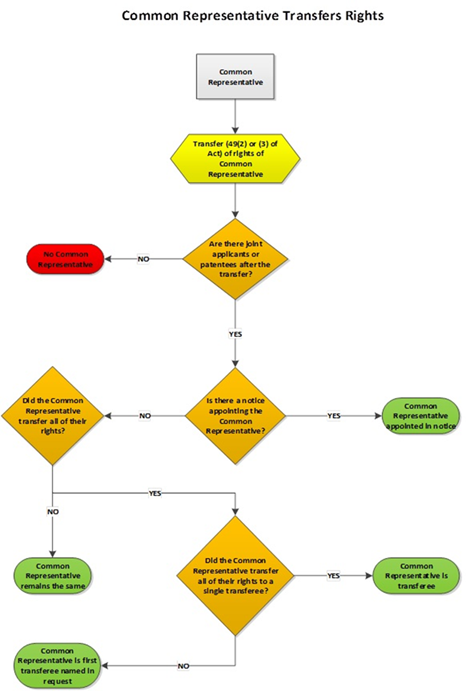
Date modified: June 2021
Any applicant, patentee or third party may appoint one patent agent, or all of the patent agents at a given firm to represent them in their business before the Patent Office. Examples of third party activities that may benefit from appointing a patent agent include filing a protest against the granting of a patent, or requesting re-examination of a patent (subsection 27(1) of the Patent Rules).
Please note that from here on, the appointment of a patent agent refers to the appointment of either one agent or all of the agents at a given firm.
Date modified: June 2021
An applicant must appoint a patent agent to represent them if they are not the inventor. Similarly, if there are multiple applicants and not all of them are inventors, they must appoint a patent agent. An appointment is also required when a transfer of the rights of an application under section 49 of the Patent Act has been recorded by the Commissioner (subsection 27(2) of the Patent Rules).
Date modified: June 2021
The Commissioner will send a notice to an applicant requiring that a patent agent be appointed within three months of the date of the notice if one is required and none is appointed (subsection 31(1) of the Patent Rules). If there are joint applicants, the notice will be sent to the common representative. Failure to appoint a patent agent within the prescribed time will result in the deemed abandonment of the application.
Date modified: June 2021
Patent applicants may appoint their patent agent at the time of filing or national phase entry as described in Section 5.04.03a of this Chapter. A patent agent may also be appointed by notice at any time during the life of a patent application or patent, as described in Section 5.04.03b of this Chapter. Another person who is not a patent applicant or patentee may also appoint a patent agent by notice for their own purposes, as described in Section 5.04.03b of this Chapter. Any appointment of a patent agent will be effective on the date that the appointment is received by the Patent Office.
The appointment of a patent agent must include the patent agent’s name and postal address. If all of the agents at a firm are being appointed, the name and postal address of the firm must be provided. Furthermore, if all agents at a firm are being appointed, it is not necessary to list each of the individual agents at the firm. An indication of the intent to appoint the firm or all agents at the firm as patent agent in respect of the application, patent or other business is sufficient (section 29 of the Patent Rules).
Date modified: October 2019
For non-PCT applications, the appointment of a patent agent may be made in the petition if the application includes a petition on its filing date. For PCT national applications, the appointment may be made in a notice submitted to the Commissioner on or before the national phase entry date. For divisional applications, the appointment may be made in the petition that is included with the application on its presentation date (paragraphs 27(3)(b), (c) and (d) of the Patent Rules). These appointments do not require the signature of the single applicant or the common representative.
Date modified: June 2021
At any time, a patent agent may be appointed in a notice to the Commissioner. For applicants and patentees who are appointing a patent agent in respect of their application or patent, the notice must be signed by the applicant or patentee or by a registered foreign practitioner who is authorized to appoint a patent agent. If the notice is signed by a registered foreign practitioner, a document authorizing that registered foreign practitioner to appoint a patent agent must also be submitted along with the notice. This authorizing document must be signed by the applicant or patentee., In cases where there are joint applicants or joint patentees, the notice must be signed by the common representative or by a registered foreign practitioner who is authorized to appoint a patent agent. If the notice is signed by a registered foreign practitioner, a document authorizing that registered foreign practitioner to appoint a patent agent must also be submitted along with the notice. This authorizing document must be signed by the common representative. (paragraph 27(3)(a) of the Patent Rules).
A person who is appointing a patent agent in respect of any other business before the Office may sign the notice appointing a patent agent themselves or may authorize a registered foreign practitioner to do so on their behalf. If a registered foreign practitioner is signing the notice, a document authorizing them to appoint a patent agent, signed by the person on whose behalf they are appointing the agent, must also be submitted along with the notice (subsection 27(4) of the Patent Rules).
Date modified: June 2021
The patent agent must consent to their appointment. If the document effecting the appointment of agent (petition, request to enter the PCT national phase, notice of appointment of agent) is submitted by the agent, then the agent’s consent is implied and evidence is not required. If the appointment of the agent is submitted by somebody other than the agent, the appointment will not be effective until evidence of the agent’s consent is received (subsection 27(5) of the Patent Rules). A letter written by the agent stating that they agree to the appointment is considered to be evidence of the agent’s consent to the appointment. If all of the agents at a firm are appointed, the letter may be written by any of the agents at the firm. This letter may be generic in nature, and no signature is required. An electronic communication from the agent to the applicant expressing consent also constitutes evidence of consent. Evidence of the agent’s consent must be submitted along with the appointment in order to avoid non-compliance with the Patent Rules.
Date modified: September 2020
The appointment of a patent agent to represent applicants is deemed to also have been made for the resulting patent, unless the appointment states otherwise (subsection 27(6) of the Patent Rules). However, in order to avoid potential errors by the Patent Office in keeping track of appointments that are limited to the application phase, the Patent Office strongly encourages applicants and patentees to submit instructions to the Patent Office at the time they wish the appointment to cease/be revoked in order for the Patent Office to give effect to the revocation.
Date modified: October 2019
In accordance with section 30 of the Patent Rules, a patent agent appointed to represent the applicant or patentee is deemed also to have been appointed to represent a transferee when a transfer of an application or a patent is recorded by the Commissioner under section 49 of the Patent Act unless the request to record the transfer states otherwise.
Date modified: June 2021
The appointment of a patent agent may be revoked by way of a notice submitted to the Commissioner. For applicants and patentees who are revoking the appointment of a patent agent made in respect of their patent application or patent, this notice must be signed by the agent (any agent if the appointment pertains to all agents at a firm), or the applicant or patentee, or by a registered foreign practitioner who is authorized to revoke the appointment. If the notice is signed by a registered foreign practitioner, a document authorizing that registered foreign practitioner to revoke the appointment must also be submitted along with the notice. This authorizing document must be signed by the applicant or patentee. In cases where there are joint applicants or joint patentees, the notice must be signed by the common representative or by a registered foreign practitioner who is authorized to revoke the appointment. If the notice is signed by a registered foreign practitioner, a document authorizing that registered foreign practitioner to revoke the appointment must also be submitted along with the notice. This authorizing document must be signed by the common representative (subsection 27(7) of the Patent Rules).
A person who is revoking the appointment of a patent agent in respect of any other business before the Office may sign the notice revoking the appointment themselves or may authorize a registered foreign practitioner to do so on their behalf. If a registered foreign practitioner is signing the notice, a document authorizing them to revoke the appointment, signed by the person on whose behalf they are revoking the appointment, must also be submitted along with the notice (subsection 27(8) of the Patent Rules).
An appointment of one patent agent is automatically revoked if the agent’s licence is suspended, revoked or surrendered by the College of Patent Agents and Trademark Agents. An appointment of all of the patent agents at a firm is automatically revoked if the licences of all of the agents at the firm are suspendered, revoked or surrendered by the College (subsections 27(7) and (8) of the Patent Rules).
Date modified: June 2021
If all of the patent agents at a firm are appointed as the patent agent in respect of a patent application, patent or other business before the Office, the following provisions apply as per section 28.1 of the Patent Rules:
Date modified: June 2021
An associate patent agent is a patent agent appointed by the appointed patent agent. A patent agent may appoint one patent agent, or all of the patent agents at the same firm to act as their associate patent agent in respect of a patent application, patent or other business before the Patent Office. (subsection 28(1) of the Patent Rules).
An appointment of an associate agent refers to an appointment of either one patent agent or all of the patent agents at a given firm as associate patent agent. Furthermore, the appointment of the associate patent agent can be made by the individual patent agent who has been appointed in respect of that application, patent or other business, or if all of the agents at the same firm have been appointed as patent agent, by any one of the patent agents at the firm.
Date modified: June 2021
An associate patent agent may be appointed at the time of filing or national phase entry, as described in Section 5.05.01a of this Chapter. An associate patent agent may also be appointed by notice at any time during the life of a patent application or patent, or other business before the Office, as described in Section 5.05.01b of this Chapter. Any appointment of an associate patent agent will be effective on the date that the appointment is received by the Patent Office.
The appointment of an associate patent agent must include the associate patent agent’s name and address. If all of the agents at a firm are being appointed, the name and postal address of the firm must be provided. Furthermore, if all agents at a firm are being appointed, it is not necessary to list each of the individual agents at the firm. An indication of the intent to appoint the firm or all agents at the firm as the associate patent agent in respect of the application, patent or other business is sufficient (section 29 of the Patent Rules).
Date modified: September 2020
For non-PCT applications, the appointment of an associate patent agent may be made in the petition if the application includes a petition on its filing date if the petition is submitted by a patent agent. For PCT national applications, the appointment may be made in a notice submitted to the Commissioner by a patent agent on or before the national phase entry date. For divisional applications, the appointment may be made in the petition that is included with the application on its presentation date if the petition is submitted by a patent agent. For clarity, the agent who is being appointed as associate patent agent in respect of the application may submit the documents themselves (paragraphs 28(3)(b)(c) and (d) of the Patent Rules). These appointments do not require a signature.
Date modified: June 2021
At any time, an associate patent agent may be appointed by the patent agent in a notice to the Commissioner signed by the appointed patent agent (subsection 28(3)(a) of the Patent Rules). If all of the patent agents at a firm are appointed as the patent agent in respect of the patent application, patent or other business, any of the agents at that firm may sign the notice appointing the associate patent agent.
Date modified: September 2020
In accordance with subsection 28(4) of the Patent Rules, the appointment of an associate patent agent by the appointed patent agent is deemed to also have been made for the resulting patent, unless the appointment states otherwise. However, in order to avoid potential errors by the Patent Office in keeping track of appointments that are limited to the application phase, the Office strongly encourages agents to submit instructions to the Office at the time they wish the appointment to cease/be revoked in order for the Patent Office to give effect to the revocation.
Date modified: June 2021
The appointment of an associate patent agent may be revoked by way of a notice submitted to the Commissioner, signed by the appointed patent agent, or the associate patent agent. If all of the patent agents at a firm are appointed as the patent agent in respect of the application, patent or other business before the Office, any of those agents may sign the notice of revocation. Similarly, if all of the patent agents at a firm are appointed as the associate patent agent, any agent at that firm may sign the notice of revocation.
The appointment of an associate patent agent is automatically revoked when the appointment of the patent agent is revoked (see section 5.04.06 of this chapter).
An appointment of one associate patent agent is automatically revoked if the associate agent’s licence is suspended, revoked or surrendered by the College of Patent Agents and Trademark Agents. An appointment of all of the patent agents at a firm as the associate patent agent is automatically revoked if the licences of all of the agents at the firm are suspendered, revoked or surrendered by the College (subsection 28(5) and (5.1) of the Patent Rules).
Date modified: June 2021
If all of the patent agents at a firm are appointed as the associate patent agent in respect of a patent application, patent or other business before the Office, the following provisions apply as per section 28.1 of the Patent Rules:
Date modified: June 2021
When a patent agent withdraws from practice, another patent agent may establish themselves as the successor to the withdrawing patent agent under section 32 of the Patent Rules. Note that for the purpose of a succession, the agent withdrawing from practice may be one individual patent agent, or all of the patent agents at a particular firm. The successor patent agent may be one individual patent agent or all of the patent agents at a given firm.
In the case of a succession, the successor patent agent is deemed to be appointed as the patent agent or the associate patent agent, as applicable, in respect of any patent application or patent in respect of which the withdrawing agent was previously appointed. To request a succession under section 32 of the Patent Rules, the successor agent must provide the following documentation to the Patent Office:
• a statement that the successor agent is the successor to the withdrawing agent pursuant to section 32 of the Patent Rules
Upon receipt of the above documentation, the Patent Office will indicate the successor agent as the appointed patent agent, or associate patent agent as applicable, in respect of the impacted patent applications and patents.
Please note that unless the Office receives instructions to the contrary, the succession will be applied to all patent applications and patents in respect of which the withdrawing agent was previously appointed, including any applications that are beyond the period of reinstatement, and any patents that have lapsed or expired. If the successor agent’s intention is to be appointed only in respect of certain patent applications and patents, a list of those applications and patents should be provided along with the succession document. If the withdrawing agent has multiple successor agents, each of the successor agents should send the above documentation to the Patent Office, and include a list indicating which patent applications and patents the successor agent intends to assume responsibility for.
Date modified: October 2019
This Chapter describes the role of certain persons, such as common representatives, patent agents and associate patent agents, who may represent patent applicants and patentees before the Patent Office. The remainder of this Chapter outlines which specific actions may be taken by whom, in respect of patent applications, and in respect of patents.
Date modified: June 2021
If there is a patent agent appointed to represent the applicant(s), or if there is a requirement to appoint a patent agent, the appointed patent agent must take action on behalf of the applicant(s) during the prosecution phase of the application. This appointed patent agent could be either the patent agent, or the associate patent agent. Note that if all of the patent agents at a firm are appointed as the patent agent or as the associate patent agent, any of the individual agents at the firm may represent the applicant(s) during the prosecution phase.
If a patent agent is not required and there is a single applicant/inventor, the single applicant must represent themselves during the prosecution phase, and if there are joint applicants/inventors, they must be represented by the common representative(subsection 36(1) of the Patent Rules).
While there are exceptions to this provision (see sections 5.07.01a and 5.07.02 of this Chapter), it is important to note that some major actions, such as the following actions, must be taken by the appointed patent agent if one is appointed or if there is a requirement to appoint one. If no agent is appointed and there is no requirement to appoint one, the following actions must be taken by the single applicant or common representative (in cases where there are joint applicants):
Date modified: June 2021
There are exceptions provided in the Patent Rules where the applicant(s) may represent themselves and where other persons may represent the applicant(s), even if a patent agent is appointed to represent the applicant(s).
Note: Some actions with respect to applications are permitted by ‘persons authorized’ by an applicant, patentee, or common representative. In these cases, the Office does not require evidence or proof of that authorization and will implicitly assume that the person is authorized. Additionally, please note that in these cases a person authorized by the common representative may be another joint applicant who is not the common representative.
The Office does require proof of authorization when a registered foreign practitioner is signing the appointment of a patent agent on behalf of the applicant(s), signing a small entity declaration, or requesting an interview with a patent examiner (subsections 27, 44 and 39 of the Patent Rules).
Date modified: June 2021
The following sections detail actions and the persons that are authorized to represent applicants with respect to patent applications. Please note that where the following sections refer to “the appointed patent agent”:
Date modified: June 2021
The following persons may file an application, pay the application fee, or in the case of PCT national phase applications, satisfy the requirements for national entry and pay the associated fees (subsection 36(2) of the Patent Rules):
Date modified: June 2021
The following persons may pay maintenance fees for applications, and the corresponding late fees, if applicable (subsection 36(2) of the Patent Rules):
Note: to see who may pay annual maintenance fees with respect to a patent, please see Section 5.07.03a of this Chapter.
Date modified: June 2021
All other fees related to a patent application, such as the final fee, may be paid by (subsection 36(5) of the Patent Rules):
Date modified: June 2021
A small entity declaration may be signed by :
Note: to see who can sign a small entity declaration with respect to a patent, please see Section 5.07.03d of this Chapter.
Date modified: June 2021
Where an application has been deemed abandoned for failure to pay a maintenance fee, the necessary steps to reinstate the application (outlined in paragraph 73(3)(a) of the Patent Act) may be taken by the appointed patent agent or by the single applicant or common representative (subsection 36(5) of the Patent Rules).
Date modified: June 2021
If there is a single applicant, a request by the applicant to record the transfer of the application under subsection 49(2) of the Patent Act may be submitted by the following persons (subsection 36(3) of the Patent Rules):
If there are joint applicants and the rights of a single joint applicant are being transferred, a request by the applicants to record the transfer of the application under subsection 49(2) of the Patent Act may be submitted by the following persons (subsection 36(3) of the Patent Rules):
If there are joint applicants and the rights of multiple joint applicants are being transferred, a request by the applicants to record the transfer of the application under subsection 49(2) of the Patent Act may be submitted by the following persons (subsection 36(3) of the Patent Rules):
Note: when a request to record the transfer of an application is made by the transferee, the transferee may represent themselves or be represented by any person authorized by them.
Date modified: June 2021
If there is a single applicant, a request to record a name change under section 125 of the Patent Rules may be submitted by the following persons (subsection 36(4) of the Patent Rules):
If there are joint applicants, a request to record a name change under section 125 of the Patent Rules may be submitted by the following persons (subsection 36(4) of the Patent Rules):
Date modified: June 2021
A reference to a previously filed application (section 27.01 of the Patent Act), or an addition to the specification of the application or additional drawing (section 28.01 of the Patent Act), may be submitted by the appointed patent agent or by the single applicant or common representative (subsection 36(5) of the Patent Rules).
Date modified: June 2021
If a patent agent has been appointed, the following persons may conduct an interview with a patent examiner (section 39 of the Patent Rules):
If a patent agent has not been appointed and is not required to be appointed (section 39 of the Patent Rules):
Date modified: October 2022
If the requested correction is being made under section 104 or subsection 154(6) of the Patent Rules, the person who submitted the application or paid the basic national fee required to enter the PCT national phase must request correction of the applicant’s identity. To see who may submit a patent application, please see Section 5.07.02a of this Chapter.
Other types of corrections to the name or identity of an applicant or inventor must be made by:
For further information on corrections to the name or identity of the applicant, please refer to Section 6.03 of Chapter 6.
Date modified: June 2021
The following tables summarize the provisions regarding representation of patent applicants:
If no patent agent is appointed and there is no requirement to appoint a patent agent:
|
|
Single applicant or common representative |
Any applicant |
Registered foreign practitioner |
Any person authorized by single applicant or common representative |
Any person authorized by any applicant |
|---|---|---|---|---|---|
|
File application and pay filing fee |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Request national phase entry and pay fee |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Pay maintenance fees |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Pay other fees |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Request Examination |
Any person may request examination[4] |
||||
|
Sign small entity declaration |
✔ |
✔ |
✔[5] |
✕ |
✕ |
|
Reinstate application abandoned for failure to pay maintenance fee |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Reinstate application abandoned for any reason other that failure to pay maintenance fee |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Request recording of transfer[6] |
✔[7] |
✕ |
✔ |
✔ |
✕ |
|
Request name change |
✔ |
✕ |
✔ |
✔ |
✕ |
|
Submit reference to previously filed application (s. 27.01 of Patent Act) |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Submit addition to specification or drawings (s. 28.01 of Patent Act) |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Interview with examiner |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Correct name/identity of applicant under s. 104 or s. 154(6) of Patent Rules |
Correction must be requested by the person who submitted the application or paid the basic PCT national phase entry fee |
||||
|
Other type of correction to name of applicant |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Other activities (ex. responding to examiner’s requisition or compliance notice) |
✔ |
✕ |
✕ |
✕ |
✕ |
If a patent agent has been appointed or there is a requirement to appoint a patent agent:
|
|
Appointed patent agent |
Single applicant or common representative |
Any applicant |
Registered foreign practitioner |
Any person authorized by single applicant or common representative |
Any person authorized by any applicant |
|---|---|---|---|---|---|---|
|
File application and pay filing fee |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Request national phase entry and pay fee |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Pay maintenance fees |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Pay other fees |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Request Examination |
Any person may request examination[8] |
|||||
|
Sign small entity declaration |
✔ |
✔ |
✔ |
✔[9] |
✕ |
✕ |
|
Reinstate application abandoned for failure to pay maintenance fee |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Reinstate application abandoned for any reason other than failure to pay a maintenance fee |
✔ |
✕ |
✕ |
✕ |
✕ |
✕ |
|
Request recording of transfer[10] |
✔ |
✔[11] |
✕ |
✔ |
✔ |
✕ |
|
Request name change |
✔ |
✔ |
✕ |
✔ |
✔ |
✕ |
|
Submit reference to previously filed application (s. 27.01 of Patent Act) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Submit addition to specification or drawings (s. 28.01 of Patent Act) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Interview with examiner |
✔ |
✔[12] |
✕ |
✔[13] |
✕ |
✕ |
|
Correct name/identity of applicant under s. 104 or s. 154(6) of Patent Rules |
Correction must be requested by the person who submitted the application or paid the basic PCT national entry fee |
|||||
|
Other type of correction to name of applicant |
✔ |
✕ |
✕ |
✕ |
✕ |
✕ |
|
Other activities (ex. responding to examiner’s requisition or compliance notice) |
✔ |
✕ |
✕ |
✕ |
✕ |
✕ |
Date modified: June 2021
Once a patent is granted, a single patentee may represent themselves or be represented by any person authorized by them. Joint patentees may be represented by the common representative or by any person authorized by the common representative (subsection 37(1) of the Patent Rules). Note that for patents, an authorized person may include, but is not limited to, an appointed patent agent or associate patent agent.
The Patent Rules provide a few exceptions to the above , which are outlined in the following sections. Please note that in cases where actions with respect to patents may be taken by ‘persons authorized’ by the single patentee, a joint patentee or the common representative who are not the appointed patent agent, the Office does not require evidence or proof of that authorization and will implicitly assume that the person is authorized. Additionally, a person authorized by the common representative may be another joint patentee who is not the common representative.
The Office does require proof of authorization when a registered foreign practitioner is signing the appointment of a patent agent or a small entity declaration on behalf of the patentee(s) (sections 28 and 112 of the Patent Rules).
Where the following sections refer to “the appointed patent agent”:
Date modified: June 2021
The following persons may pay maintenance fees for patents, and the corresponding late fees, if applicable (subsection 37(1) of the Patent Rules):
Note: to see who may pay annual maintenance fees with respect to a patent application, please see Section 5.07.02b of this Chapter.
Date modified: June 2021
If there is a single patentee, a request by the patentee to record the transfer of the patent under subsection 49(3) of the Patent Act may be submitted by the following persons (paragraph 37(1)(a) and subparagraph 37(1)(b)(ii) of the Patent Rules):
If there are joint patentees and the rights of a single joint patentee are being transferred, a request by the patentees to record the transfer of the patent under subsection 49(3) of the Patent Act may be submitted by the following persons (paragraph 37(1)(a) and subparagraph 37(1)(b)(ii) of the Patent Rules):
If there are joint patentees and the rights of multiple joint patentees are being transferred, a request by the patentees to record the transfer of the patent under subsection 49(3) of the Patent Act may be submitted by the following persons (paragraph 37(1)(a) and subparagraph 37(1)(b)(ii) of the Patent Rules):
Note: When a request to record the transfer of the rights to a patent is made by the transferee, the transferee may represent themselves or be represented by any person authorized by them.
Date modified: December 2025
The following persons may take action for the purposes of making observations under subsection 117.11(7) of the Patent Rules (reconsideration of additional term), the grant of an additional term under section 46.1 of the Act, the reissuance a patent under section 47 of the Patent Act, making a disclaimer under section 48 of the Patent Act, filing a reply under subsection 48.2(5) of the Patent Act, or participating in a process under section 48.3 of the Patent Act with respect to re-examination (subsection 37(2) of the Patent Rules):
Date modified: December 2025
A small entity declaration may be signed by:
With respect to a request for re-examination, a small entity declaration may be signed by:
Note: to see who may sign a small entity declaration with respect to a patent application, please see Section 5.07.02d of this Chapter.
The following table summarizes the provisions regarding representation of patentees:
Representation of Patentees – who can act?
Whether or not a patent agent is appointed:
|
|
Appointed patent agent |
Single patentee or common representative |
Any patentee |
Registered foreign practitioner |
Any person authorized by single patentee or common representative |
Any person authorized by any patentee |
|---|---|---|---|---|---|---|
|
Pay maintenance fees |
✔ |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Sign small entity declaration |
✔ |
✔ |
✔ |
✔[14] |
✕ |
✕ |
|
Request recording of transfer[15] |
✔ |
✔[16] |
✕ |
✔ |
✔ |
✕ |
|
Granting of additional term (including making observations) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Making observations (reconsideration of additional term) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Reissue a patent |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Make a disclaimer |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
File a reply (s. 48.2(5) of Patent Act) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Participate in re-examination proceeding (s. 48.3 of Patent Act) |
✔ |
✔ |
✕ |
✕ |
✕ |
✕ |
|
Other activities |
✔ |
✔ |
✕ |
✔ |
✔ |
✕ |
Date modified: January 2025
The Office will send all correspondence regarding a patent application or a patent to the following person:
If no patent agent appointed, the Office will send all correspondence to:
Exceptions will be made in the following circumstances:
Date modified: June 2021
The Office will have regard to written communications from persons authorized to represent applicants and patentees outlined in sections 33 to 37 of the Patent Rules. Written communications from others will be disregarded, except in the following cases:
In these three circumstances, the Office will respond to the person with a notice of disregarded communication. In all three cases, the person will be given an opportunity to comply with the Patent Rules and have the Office give regard to their original communication. Details are provided in Sections 5.09.01, 5.09.02 and 5.09.03 of this Chapter.
Date modified: January 2025
If the Patent Office receives a communication from a joint applicant or joint patentee who is not the common representative, for a purpose for which the common representative may represent the joint applicants or joint patentees, the Office must send a notice of disregarded communication, under subsection 40(1) of the Patent Rules, to the joint applicant or joint patentee. A copy of the notice will be sent to the common representative of the joint applicants or joint patentees (subsection 40(1.1) of the Patent Rules).
The notice of disregarded communication indicates that the Office will not have regard to the communication, unless within three months of the date of the notice the joint applicant or joint patentee is appointed as common representative and requests that the Commissioner have regard to their initial communication.
If the joint applicant or joint patentee is then appointed as common representative before the expiry date of the notice (by notice submitted to the Commissioner in compliance with paragraph 26(3)(a) of the Patent Rules), and makes the necessary request for the Commissioner to have regard to that communication, the original communication is deemed to have been received from the common representative on the day that it was received at the Patent Office (subsection 40(3) of the Patent Rules). Note that these provisions do not apply to communications for purposes which may be carried out by any joint applicant or joint patentee or by persons authorized by the common representative or a joint applicant or joint patentee (subsection 40(2) of the Patent Rules).
Date modified: January 2025
The Office will send a notice of disregarded communication to a patent agent who communicates with the Office on behalf of an applicant or patentee but is not appointed as the patent agent or associate patent agent for that applicant or patentee in respect of that application or patent. The notice indicates that the Office will not have regard to the patent agent’s communication unless within three months of the date of the notice, the patent agent is appointed as agent or associate agent in respect of that application or patent and requests that the Office have regard to their initial communication (subsection 41(1) of the Patent Rules). A copy of the notice will be sent to the default correspondent (see section 5.08 of this Manual), who is considered the applicant or patentee for the purposes of subsection 41(1.1) of the Patent Rules.
If the patent agent is then appointed as agent or associate agent in respect of that application or patent (by notice submitted to the Commissioner in compliance with paragraph 27(3)(a) or 28(3)(a) of the Patent Rules), and makes the necessary request for the Commissioner to have regard to the communication, the original communication is deemed to have been received from the applicant or patentee on the date that it was received at the Office (subsection 41(3) of the Patent Rules). Note that these provisions do not apply to communications for purposes which may be carried out by any person who is authorized by an applicant or patentee (subsection 41(2) of the Patent Rules).
Date modified: January 2025
If the Patent Office receives a communication on behalf of an applicant or patentee that appears to come from a patent agent, but the name of a patent agent is not provided, the Office will send a notice of disregarded communication to the person, indicating that the Commissioner will not have regard to the communication unless within three months of the date of the notice, the person writes to the Commissioner providing their name and requests that the Commissioner have regard to the original communication (subsection 41.1(2) of the Patent Rules). A copy of the notice will be sent to the default correspondent (see section 5.08 of this Manual), who is considered the applicant or patentee for the purposes of subsection 41(2.1) of the Patent Rules.
If these actions are taken within the timeframe provided, and if the person was a patent agent on the date that the communication was submitted, the communication will be deemed to have been received from the patent agent on the date that it was originally received by the Commissioner (subsection 41.1(3) of the Patent Rules).
Note that the above provisions do not apply to communications for purposes which may be carried out by any person who is authorized by an applicant or patentee. For example, if all agents at a firm are appointed, and a maintenance fee payment is submitted by a person at that firm who is not a patent agent, the Office will accept the payment without sending a notice, because any person authorized by an applicant or patentee may pay a maintenance fee (subsections 36(2) and 37(1) of the Patent Rules). The name of a patent agent is therefore not required. However, if a response to an examiner’s report or a final fee payment is submitted by the firm without specifying the name of a patent agent at the firm, the above provisions would apply and a notice of disregarded communication would be sent to the firm. In these cases the Office would need to ensure that the person from the firm who submitted the communication is indeed a patent agent, in order to ensure that the submission accords with the Patent Rules’ provisions on representation of patent applicants (subsections 36(1) and 36(5) of the Patent Rules).
Date modified: September 2020
A patent for an invention confers a property right on the inventor or in some cases on an employer of an inventor where the invention was made in the normal course of employment. For more information on establishing entitlement to apply for a patent, please see Section 4.04 in Chapter 4.
The rights to a patent application or a patent may be transferred to another person at any time. The Commissioner will record the transfer upon receipt of a request compliant with section 126 of the Patent Rules, and with payment of the prescribed fee listed on CIPO’s webpage of Patent Fees.
The history of transferring or passing on the right to a patent or an application is called the chain of title. The chain of title reflects any request, subject to the Patent Act and the Patent Rules, that transfers ownership from the original applicant or any subsequent changes of owner.
Date modified: October 2019
Patents can be sold, licensed or used to negotiate funding, venture capital or other forms of financing. While it is not mandatory for patent applicants or patentees to record a transfer or a change of name with the Patent Office, there are benefits to doing so in a timely manner. It allows the Patent Office to issue patents to the correct owners of the rights accorded by the patent and maintain accurate records. It also ensures that the Patent Office’s records are up to date for the public to easily recognize who owns the rights to the invention.
The Patent Office recommends that persons submitting requests under section 126 of the Patent Rules use the form provided.[17] This will help ensure that all the necessary information is provided and will expedite the processing of these requests.
Date modified: September 2020
Pursuant to subsection 31(3) of the Patent Act, where an application is filed by joint applicants and it subsequently appears that one or more of them has had no part in the invention, the prosecution of the application may be carried on by the remaining applicant or applicants on satisfying the Commissioner by affidavit that the remaining applicant or applicants is or are the sole inventor or inventors (or their legal representatives).
Pursuant to subsection 31(4) of the Patent Act, where an application is filed by one or more applicants and it subsequently appears that one or more further applicants should have been joined, the further applicant or applicants may be joined on satisfying the Commissioner that they should be so joined, and that the omission of the further applicant or applicants had been by inadvertence or mistake and was not for the purpose of delay.
Date modified: October 2019
Pursuant to section 52 of the Patent Act, the Federal Court has jurisdiction, on the application of the Commissioner or of any person interested, to order that any entry in the records of the Patent Office relating to the title to a patent be varied or expunged, including the removal of a previously registered document.
Date modified: October 2019
Inventions are frequently created as part of a collaborative effort. In such instances, all the inventors must join in applying for a patent.
Pursuant to subsection 31(1) of the Patent Act, if one of the inventors refuses to apply for a patent or his whereabouts cannot be ascertained after diligent inquiry, the other inventors or their legal representatives may apply for a patent, and a patent may be granted in the name of the inventors who apply for a patent on satisfying the Commissioner that the joint inventor has refused to apply for a patent or that his whereabouts cannot be ascertained after diligent inquiry.
Date modified: October 2022
When amendments are made, such that the subject-matter of the invention for which an exclusive privilege or property is claimed changes, the inventors listed for the application may also need to be updated. Inventors can be added or removed upon request. Such changes will be processed under section 106(b) of the Patent Rules so long as the request is received before the day on which the notice of allowance or conditional notice of allowance is sent. Note that the Office will not evaluate any evidence regarding ownership of the patent or patent application in the case of a disagreement between inventors and/or applicants.
Date modified: October 2022
One of the most common errors in patent applications is the naming of applicants. For that reason, the Patent Office encourages clients to review all documents prior to submission to ensure that they are error free. The following sections detail how to correct errors in the naming of applicants. Errors in the naming of applicants might require a correction of the applicant identity or the name of the applicant.
Note that corrections are distinct from the recording of transfers of rights or changes of name. For more information, please see sections 6.05 – 6.07.
If the applicant is also an inventor, a separate request to correct an error in the naming of the inventor will need to be submitted. Note that the timelines outlined in the Patent Rules to do so for corrections to applicant identities is shorter than those for inventor identities.
Please note that no fee is required for correcting an error in the naming of an applicant during the application stage.
Date modified: October 2022
Where the error in naming results in an applicant being incorrectly identified at the time a patent application is filed or at the time a PCT application enters the national phase, there is a small window during which the identity can be corrected when the error was due to inadvertence, accident or mistake without any fraudulent or deceptive intention. In other words, when the person named as applicant has no rights to the invention, the Patent Rules provide mechanisms to replace the incorrect individual with the correct applicant.
For example, Bob was named as an applicant at the time the application was filed but Bob has no rights to the invention. The person who submitted the application incorrectly identified Bob when he should have identified John as the applicant.
A correction of name is meant to correct errors that do not change the identity such as incorrect spellings. For example, Bob was named as the applicant at the time the application was filed and he has the rights to the invention. Bob is a nickname and he should have been identified as Robert.
The Office is generally not in a position to be able to determine whether a correction of an applicant name corrects the identity or only the name of that person. Therefore, persons submitting a correction request to the Office should clearly note the type of correction (identity or name only) and it will generally be treated accordingly.
Date modified: October 2019
The person who submitted the application to obtain a filing date or for PCT national phase entry will be able to request the correction of the identity of the applicant, as long as the error arose from inadvertence, accident or mistake without any fraudulent or deceptive intention and the person who submitted the application provides a statement to that effect.
Date modified: October 2019
For a regularly filed application the request will have to be made before the earlier of when the application becomes open to public inspection and the day on which the Commissioner receives a request to record a transfer under section 49 of the Patent Act (section 104 of the Patent Rules).
Date modified: October 2019
For a PCT national phase application the request must be submitted before the earlier of (subsection 154(6) of the Patent Rules):
Date modified: October 2022
An error in the naming of an applicant that does not result in a change of identity can be corrected by request from the applicant so long as it is submitted on or before the payment of the final fee, or if the final fee is refunded, on or before it is paid again. The request must also contain a statement to the effect that the correction does not add or delete the name of an applicant or change the identity of a named applicant (section 105 of the Patent Rules).
Date modified: October 2022
All requests for correction to the naming of an applicant in a patent application must comply with the requirements for submitting written communications to the Commissioner. Every request must include:
(see Section 2.02.01 in Chapter 2 for more information)
Date modified: September 2020
The correction of an error in a patent application will result in updating office records and the requester will be informed by letter that the correction has been made if requested by the applicant.
Date modified: October 2022
One of the most common errors in patent applications is the naming of inventors. For that reason, the Patent Office encourages clients to review all documents prior to submission to ensure that they are error free. The following sections detail how to correct errors in the naming of inventors. Errors in the naming of inventors might require a correction of the inventor identity or the name of the inventor.
A correction of inventor identity is when the wrong person was identified in the application. For example, the wrong person, Bob, was named as inventor, when it should have been John.
A correction of name is meant to correct errors that do not change the identity such as incorrect spellings. For example, Bob was named as the inventor. Bob is a nickname and he should have been identified as Robert.
The Office is generally not in a position to be able to determine whether a correction of an inventor name corrects the identity or only the name of that person. Therefore, persons submitting requesting a correction request to the Office should clearly note the type of correction (identity or name only) and it will generally be treated accordingly.
If the inventor is also an applicant, a separate request to correct an error in the naming of the applicant will need to be submitted. Note that the timelines outlined in the Patent Rules to do so for corrections to applicant identities is shorter than those for inventor identities.
Note that corrections are distinct from changes of name. For more information, please see Section 6.07 of this Chapter.
Please note that no fee is required for correcting an error in the naming of an inventor during the application stage.
Date modified: October 2022
An incorrectly identified inventor for a patent application can be corrected by request from the applicant if it is submitted before a notice of allowance or conditional notice of allowance is sent (section 106 of the Patent Rules).
Date modified: October 2022
An error in the naming of an inventor in a patent application that does not result in a change of identity can be corrected by request from the applicant so long as it is submitted on or before the payment of the final fee, or if the final fee is refunded, on or before it is paid again. The request must also contain a statement to the effect that the correction does not add or delete the name of an inventor or change the identity of a named inventor (section 106 of the Patent Rules).
Date modified: June 2023
All requests for corrections to the naming of an inventor in a patent application must comply with the requirements for submitting written communications to the Commissioner. Every request must include:
(see Section 2.02.01 of Chapter 2 for more information)
Date modified: October 2019
The correction of an error in a patent application will result in updating office records and the requester will be informed by letter that the correction has been made.
Date modified: January 2024
In order to process the request correctly under the Patent Act and Patent Rules, applicants must clearly indicate what is being requested of the Commissioner and preferably indicate the section of the Patent Act or Patent Rules the office is to consider when processing your request.
For example, consider the situation where an applicant sends correspondence to the Commissioner that includes the following:
In this scenario, it would not be clear whether this is a request for registration of a related document under section 124 of the Patent Rules, a request to change the name of the applicant under section 125 of the Patent Rules, or a request to record a transfer under section 126 of the Patent Rules, or a combination of requests.
In cases where it is not clear from correspondence which request is being made, the Office will send a letter asking the requestor for clarification. In order to avoid delays in processing your request, it is recommended that requests include clear instructions and references to the appropriate sections of the Patent Act or Patent Rules.
Date modified: October 2019
A patent grants the patentee and the patentee’s legal representatives the exclusive right, privilege and liberty of making, constructing and using the invention and selling it to others to be used. Those rights are established at the filing date when the applicant(s) make a claim to those exclusive rights through the patent application. The subsequent transfer of those rights from one applicant/patentee may be recorded by the Commissioner following a request under section 49 of the Patent Act. Note that the effective date for the recording is the date when the Commissioner records the transfer and not when the request is submitted to the Patent Office.
Date modified: September 2020
Although subsection 49(1) of the Patent Act states that the right or interest in an invention is transferable, section 49 does not provide for the recording of such transfers.
If a request is submitted for the recording of a transfer of a right or interest in an invention to a person who is currently recorded as an applicant in the records of the Patent Office, the Office will notify the requestor that the transfer cannot be recorded under section 49 of the Patent Act.
If a request is submitted for the recording of a transfer of a right or interest in an invention to a person who is not currently recorded as an applicant in the records of the Patent Office, the Office will notify the requestor that section 49 of the Patent Act does not provide for the recording of rights or interest in an invention.
If a request is incorrectly submitted for the recording of a transfer of a right or interest in an invention, a refund may be requested of any fee paid in respect of that request.
Date modified: September 2020
A transfer of an international application that took place before the national phase entry date in Canada is not considered to be a transfer of an application under section 49(2) of the Patent Act since, under subsection 155(1) of the Patent Rules, an international application is considered to be an application for a patent filed in Canada beginning only on its national phase entry date.
If a request is submitted for the recording of the transfer of an international application that took place before the national phase entry date in Canada, the Office will notify the requestor that the Commissioner has not recorded the transfer. In this case, a refund of the fee for requesting that a transfer be recorded can be requested.
Date modified: January 2024
In order to record a transfer, the submission must contain (section 126 of the Patent Rules):
All requests for corrections of a patent application must comply with the requirements for submitting written communications to the Commissioner. Please see Section 2.02.01 of Chapter 2 for more information.
Date modified: October 2019
If a request to record a transfer is submitted by the applicant or the patentee, the Commissioner will record the transfer without additional required evidence (subsections 49(2) and (3) of the Patent Act). Please see Chapter 5 for more information on who can represent the applicant or patentee.
Date modified: October 2019
If a request to record a transfer is submitted by the transferee (and not the currently recognized applicant or patentee), the transferee will need to provide evidence of the transfer to the Commissioner (subsections 49(2) and 49(3) of the Patent Act). A copy of the document effecting the transfer of rights along with one of the examples of evidence provided below would be considered satisfactory:
Please note that the request and any evidence submitted will be placed on file and be open to public inspection as required under section 10 of the Patent Act.
Please see Chapter 5 for more information on who can represent the transferee.
Date modified: October 2019
Once the Commissioner has recorded the transfer, a certificate with a unique identification number will be sent to the person who requested the recording of the transfer.
Date modified: October 2019
The Commissioner will remove the recording of the transfer of an application or a patent upon receipt of evidence satisfactory to the Commissioner that the transfer should not have been recorded. However, the Commissioner is not authorized to remove the recording of a transfer of a patent for the reason only that the transferor had previously transferred the patent to another person.
Date modified: October 2019
Changes of name that do not change the identity will be recognized on the request of the applicant or patentee. Changes of name are distinct and separate from the correction mechanisms for applicants under the Patent Rules. For more information on correction mechanisms, please refer to Sections 6.03 and 6.04 of this Chapter.
Date modified: September 2020
The Office takes the position that section 125 of the Patent Rules applies only to a name change of a person who is currently recorded as an applicant or patentee in the records of the Office. The Office will not record a name change of a previously recorded applicant or patentee.
Section 125 of the Patent Rules provides for the recording of the fact of a name change and does not provide for the registration of any related documents. If desired, however, any document relating to a patent or an application may be registered separately under section 124 of the Patent Rules.
Date modified: January 2024
In order to record a change of name, the submission must contain (section 125 of the Patent Rules):
All requests to record a change of name must comply with the requirements for submitting written communications to the Commissioner. (Please see Section 2.02.01 in Chapter 2)
Please see Chapter 5 for more information on who can represent the applicant.
Date modified: October 2019
Once the Commissioner has recorded a change of name, a certificate with a unique identification number will be sent to the person who requested the change of name.
Date modified: September 2020
Any person may submit a request to the Commissioner to register a document relating to a patent application or patent (section 124 of the Patent Rules). Note that the registration of a document is a separate mechanism than the recording of a transfer. Registration of a document, which affects a patent transfer or documents a name change, will simply put that document on file at the Patent Office. It will not be treated as a request to record a transfer or change of name. Applicants may also choose to submit the document as an attachment to correspondence to the Office and not request registration of the document. In this case, the related document will be placed on file and will be available to the public once the application is open to public inspection. A registration certificate or a registration number will not be provided.
Date modified: January 2024
In order to register a document, the submission must contain (section 124 of the Patent Rules):
Date modified: October 2019
Once the Commissioner has registered a document, a certificate with a unique identification number will be sent to the person who requested the registration of the document.
Date modified: September 2020
The Office does not take a position as to whether a merger in fact transferred the rights to the application or whether it was simply a name change that occurred through a merger.
If a merger affecting an applicant for a patent occurs, clients must decide how to manage their application. Applicants may choose to do nothing, or they may opt to make one of the following requests:
Date modified: October 2019
Pursuant to section 10 of the Patent Act most documents relating to a patent or patent application submitted by applicants and/or agents are made available on the Canadian Patents Database (CPD). All patents, applications for patents and documents relating to patents or applications for patents that are in the possession of the Patent Office become available to the public once a patent application's confidentiality period under the Patent Act has expired.
Documents and information submitted by applicants and/or agents effecting transfers or changes of names may contain sensitive personal information. CIPO will not publish these documents on the CPD, but they will be available to the public either in person at our Office in Gatineau or upon request and payment of a prescribed fee.
Submit only information needed as prescribed by the Patent Act and Rules. You may redact or suppress any information that you do not wish to be made public before you submit the document to CIPO, so long as this information is not required information according to the Patent Act and Patent Rules.
Date modified: October 2019
This chapter addresses the requirements for requesting priority from an application previously filed in Canada or in any country belonging to the Paris Convention for the Protection of Industrial Property (“the Paris Convention”) or in any World Trade Organization (WTO) member country and the mechanisms for withdrawing priority from an application.
Date modified: October 2019
Article 4 of the Paris Convention provides for the right of priority for patent applications filed in any country of the Union established by Article 1, section 1 of the Paris Convention. Article 2(1) of the WTO’s Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) provides that Members shall comply with Articles 1 through 12, and Article 19, of the Paris Convention (1967).
Claiming priority allows an applicant to benefit from a claim date that is earlier than the actual date of filing of the application. An applicant is required to file a request for priority to gain the earlier claim date. Priority is based on subject-matter disclosed in a priority document and is not restricted to what is claimed in the priority document.[18] A principal advantage provided by the right of priority is to give applicants time to decide whether they want to seek protection in one or more countries for an invention based on the filing of an earlier application (i.e. a priority document) in a country affording priority rights. This enables an applicant to disclose or publicly practice the later claimed invention between the filing of the priority document and the subsequent application. The effects of a request for priority are discussed in the context of the patentability of a claim in section 18.03 of this manual.
Date modified: October 2019
The requirements for requesting priority in respect of an application for patent regularly filed[19] in Canada are set out in section 28.4 of the Patent Act and in section 73 of the Patent Rules.
Subsection 28.4(1) of the Patent Act provides that
(1) For the purposes of sections 28.1, 28.2 and 78.3, an applicant for a patent in Canada may request priority in respect of the application on the basis of one or more previously regularly filed applications.
Date modified: October 2019
The request for priority must be made in the petition of the pending application or in another document other than the specification or drawings of the pending application.
For each previously regularly filed application (a priority application), subsection 28.4(1) of the Patent Act and 73(1) of the Patent Rules require the following information to make the request for priority:
Date modified: September 2020
Subsection 28.4(2.1) of the Patent Act provides that
(2.1) A request for priority is deemed never to have been made if the request is not made in accordance with the regulations or if the applicant does not submit the information, other than the number of each previously regularly filed application, required under subsection (2).
When the application number of the priority application is not known, the Patent Office will accept the following in the place of the application filing number:
Date modified: January 2025
Under subsection 73(1) of the Patent Rules, an applicant may make a request for priority before the earlier of:
Date modified: September 2020
While it is possible to make corrections to the information required to make a request for priority, the time period to make these corrections can be very short. Therefore the Office strongly recommends that applicants ensure that the request is free from errors before it is submitted to the Office.
If there is an error in the filing date of the priority application submitted to the Office, it may be possible to correct it if the applicant has not requested that it be opened to public inspection before the end of the confidentiality period and if the request for the correction is submitted before the earliest deadline for making a request for priority when calculated using the uncorrected date and the corrected date.
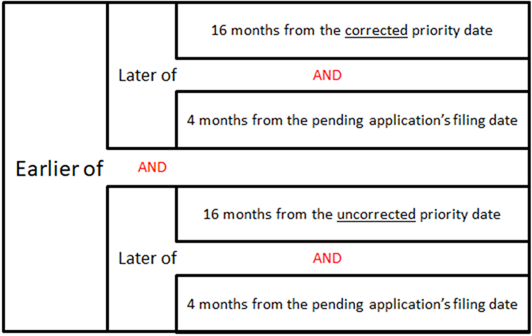
Example 1
An applicant with a filing date of November 25, 2019 made a single request for priority on that date for priority to an application whose filing date was erroneously noted as November 19, 2018. The applicant then received a filing certificate sent on December 17, 2019 and realized that their mistake and the correct filing date for the priority application is November 29, 2018. The applicant has not requested early public inspection of the patent application under subsection 10(2) of the Patent Act.
The later of 16 months from the corrected priority date (Nov 29, 2018 + 16 months = Mar 29, 2020) and 4 months from the application’s filing date (Nov 25, 2019 + 4 months = Mar 25, 2020) is Mar 29, 2020.
The later of 16 months from the uncorrected priority date (Nov 19, 2018 + 16 months = Mar 19, 2020) and 4 months from the application’s filing date (Nov 25, 2019 + 4 months = Mar 25, 2020) is Mar 25, 2020.
The earlier of these 2 results is Mar 25, 2020.
Therefore, the applicant may request a correction in the filing date of the priority application on or before March 25, 2020.
Example 2
An applicant with a filing date of February 10, 2020 made a single request for priority four months after the filing date (on June 10, 2020) to a priority application dated February 14, 2019. The applicant then received a confirmation letter sent on June 30, 2020 for the request for priority and realized that they had made an error and that the priority date should have been February 10, 2019. The applicant has not requested early public inspection of the patent application under subsection 10(2) of the Patent Act.
The later of 16 months from the requested corrected priority date (Feb 10, 2019 + 16 months = Jun 10, 2020) and 4 months from the application’s filing date (Feb 10, 2020 + 4 months = Jun 10, 2020) is Jun 10, 2020.
The later of 16 months from the originally submitted priority date (Feb 14, 2019 + 16 months = Jun 14, 2020) and 4 months from the application’s filing date (Feb 10, 2020 + 4 months = Jun 10, 2020) is Jun 14, 2020.
The earlier of these 2 results is Jun 10, 2020.
Therefore, the applicant may request a correction in the filing date of the priority application on or before Jun 10, 2020.
Date modified: October 2019
It is possible to request the correction of an error in the name of a country or office of filing and the number of the priority application under subsection 73(5) of the Patent Rules. The request must be submitted on or before payment of the final fee or if the final fee is refunded, on or before it is paid again.
Date modified: September 2020
The applicant is required to provide a copy of each priority application for which they have made a request for priority under subsection 74(1) of the Patent Rules. This can be done by either:
Date modified: September 2020
An applicant may submit a certified copy of the priority document and a certificate from the office of filing showing the filing date by all appropriate means set out in Chapter 2 of this manual (including all electronic means).
Date modified: February 2022
Under subparagraphs 67(2)(b)(ii), 72(3)(a)(ii), and paragraphs 74(1)(b), 181(1)(b) and 196(1)(b) of the Patent Rules, the World Intellectual Property Organization Digital Access Service (DAS) is specified by the Commissioner as being accepted for the purpose of making a copy of a previously filed application available to the Commissioner.
The DAS is a secure electronic digital library service, administered by WIPO to facilitate the secure exchange of patent, trademark and design priority documents between Intellectual Property Offices (IPOs). Applicants may request that the IP office that has their priority document upload an electronic copy to the DAS.
Applicants who wish to make a copy of a previously filed application available to the Commissioner in a digital library, the WIPO DAS, must provide the Office with the access code for that application. Applicants should ensure that they have taken the correct steps to have the priority application uploaded to DAS prior to providing the access code to the Office. While some intellectual priority offices automatically upload applications to the DAS, others may not upload the application unless they are specifically requested to do so.
Applicants who wish to upload a copy of a Canadian filed application or a Patent Cooperation Treaty (PCT) application that was filed with Canada working as the receiving office for use as a priority document in WIPO DAS, may make a request for CIPO to do so using the request form provided on the CIPO Order IP Documents web page.
Date modified: October 2019
Applicants are not required to provide copies of priority documents according to subsections 74(1) and (12) of the Patent Rules, under the following circumstances:
Date modified: October 2019
Applicants who are required to submit or make available copies of priority documents must do so according to subsection 74(2) of the Patent Rules, before the latest of the following dates:
Date modified: October 2019
If the applicant has not complied with the requirement to provide a copy of the priority document by the deadline outlined in 74(2) of the Patent Rules and described in Section 7.04.04 in this Chapter, the Commissioner will send a notice to the applicant under subsection 74(4) of the Patent Rules requiring the applicant to do so not later than two months after the date of the notice.
Date modified: October 2019
Applications filed before October 30, 2019 (the coming into force date of the Patent Rules [SOR/2019-251]) that contain a compliant request for priority made before that date are not required to provide a copy of the priority application.
If during the course of examination, the examiner takes into consideration the priority, they may require the applicant to submit a copy of the priority application by notice under subsection 196(1) of the Patent Rules. The applicant must submit or make available a copy of the relevant priority document(s) not later than four months after the date of the notice.
Date modified: October 2019
In the event that an applicant is unable to meet the requirement to submit a copy, or provide access to the priority document, according to subsection 74(6) of the Patent Rules, the applicant will be deemed to have complied with the requirement to submit a copy or provide access if the applicant takes the following steps:
Under subsection 74(8) of the Patent Rules, when a copy of the priority document and certificate is provided by the office of filing the applicant must submit them to the Commissioner not later than three months after the day on which they were received by the person who requested them.
Date modified: October 2019
During the examination of an application, an examiner may send a notice requiring the applicant to submit a translation of a priority document that is partly or entirely in a language other than English or French. See section 12.05.03 for further information.
If the examiner has reasonable grounds to believe that the translation is not accurate, they may send a further notice to the applicant requesting:
Date modified: October 2019
“Restoration of the right of priority” is a mechanism whereby the time limit for filing an application accompanied by a request for priority is extended beyond the normal twelve-month period after the filing of a priority document.
For regular applications filed in Canada and for PCT national phase applications, applicants can request the restoration of the right of priority when the filing date of the pending application is more than twelve months after the filing date of the previously regularly filed application, but within two months after the end of those twelve months.
Date modified: October 2019
Restoration of the right of priority is available for patent applications that have a filing date that is on or after October 30, 2019, the coming-into-force date of the Patent Rules (SOR/2019-251).
Date modified: October 2019
In order to request the restoration of priority under paragraph 28.4(6)(b) of the Patent Act and subsections 77(1) and 77(2) of the Patent Rules, the applicant must, within the relevant prescribed time set out in section 77 of the Patent Rules (see Section 7.03.02 of this Chapter):
Date modified: October 2019
The time to make a request to restore the right of priority under subsection 77(1) of the Patent Rules, is:
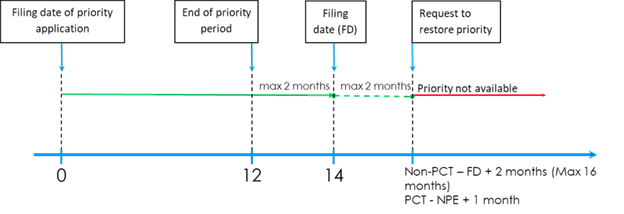
Date modified: October 2019
Restoration of the right of priority will be recognized in Canada on the national phase entry date of a PCT national phase application or a divisional application resulting from the division of a PCT national phase application under section 162 of the Patent Rules if:
Date modified: October 2019
The deemed restoration in Canada will be effective for PCT national phase applications with an international filing date that is on or after October 30, 2019, the coming-into-force date of the Patent Rules (SOR/2019-251).
Date modified: October 2019
A request for priority will be considered withdrawn under subsections 74(6), 74(9) and 76(3) of the Patent Rules in the following circumstances:
Date modified: September 2020
Under certain circumstances, an applicant may wish to withdraw a request for priority. This may be the case where, for example, the earlier application is withdrawn before it is laid open to public inspection or where the applicant determines that the later claimed subject-matter is not disclosed in the earlier application.
A request for priority may be withdrawn by the applicant upon request to the Commissioner. The effective date of the withdrawal of a request for priority is the date on which the request is received by the Commissioner.
The withdrawal of priority may have an effect on the claim date, as defined in section 28.1 of the Patent Act. If the claim date changes, this may affect what prior art documents are applicable under sections 28.2 and 28.3 of the Patent Act (see chapter 18).If the applicant withdraws a request for priority stemming from the earliest previously filed application before the expiry of the confidentiality period it may be possible to delay the laying open of the application to public inspection until eighteen months from the next earliest priority date or, where no other priority documents exist, the filing date of the application.
Withdrawing a request for priority with respect to a non-laid open public application may affect the confidentiality period. For more information, please see Chapter 8.
Date modified: October 2019
Several intergovernmental organisations exist to centralize the patent search and examination process for a number of member countries. An applicant may request priority in Canada based on a previously filed application submitted to the intergovernmental organisation.
For example, an applicant seeking priority from an application filed at the African Regional Intellectual Property Organization (ARIPO) may identify the priority document by naming ARIPO as the authority[20] and provide the filing date and application number issued by ARIPO.
Date modified: October 2019
International applications are filed before an international organisation which examines the application but does not issue a patent effective in any member state without further actions by the applicant to secure patent rights in elected states. These applications may form the basis of priority for applications filed in Canada.
Date modified: October 2019
The filing of a PCT application has the effect of filing a regular national application[21] in each state designated in the international application. The Canadian filing date of the national phase application is the same as the filing date for the corresponding PCT application. In accordance with the Paris Convention, the effect of an international application is equivalent to that of a national filing. Priority rights, for example, may be based on an international application.
For example, an international application may be filed directly with the International Bureau of WIPO. Such an application will be assigned an application number bearing the two-letter code ‘IB’. Therefore, on filing a request for priority in Canada based on the internationally filed application, the applicant will identify the International Bureau as the receiving office and provide the application number assigned by the International Bureau.
If the international application has acquired priority rights before the International Bureau on the basis of an earlier filed application, those rights would be extended to the application upon national entry in Canada except in situations where restoration of priority rights has occurred in the international phase in relation to an application whose filing date is before October 30, 2019 (see Section 7.06 in this Chapter).
Date modified: October 2019
Office acknowledges priority based on an application filed with the European Patent Office (EPO).[22]
Date modified: October 2019
While subsection 3(1) of the Patent Rules generally permits the Commissioner to grant extensions of time limits, subsection 73(7) of the Patent Rules provides that the Commissioner is not permitted to extend time limits for providing the Office information necessary to recognise a request for priority.
Date modified: October 2019
Where the twelve-month anniversary date defined in paragraph 28.1(1)(b) of the Patent Act is a prescribed or designated day under section 78 of the Patent Act, such as a day when the Patent Office is closed to the public, the filing of the pending application may be made on the next day that is not a prescribed or designated day without forfeiting priority rights.
Date modified: October 2019
Several additional considerations pertaining to valid priority rights but which are not explicitly addressed by the Patent Act and Patent Rules should be noted.
Date modified: September 2020
Where the applicant named on the priority document is different than the applicant for the pending Canadian application, the applicant in Canada is recommended to furnish the Patent Office with evidence that priority rights have been transferred in order to establish that the requirements of subparagraph 28.1(1)(a)(i) of the Patent Act have been satisfied.
Date modified: October 2019
The Patent Office recognizes Paris Convention priority based on petty patent applications, applications for inventors’ certificates[23], and utility models filed in foreign countries[24], as these are considered forms of patent applications. No priority rights for a patent application may be based on an application for an industrial design registration, design patents or their equivalent.
Date modified: October 2019
An applicant who files a patent application in Canada must pay annual maintenance fees starting at the 2nd anniversary of the filing date to maintain the application in effect according to subsection 27.1(1) of the Patent Act and section 68 of the Patent Rules.
Please see Chapter 5 for information on who can pay maintenance fees and late fees for applications.
Date modified: September 2020
The amounts and time limits for paying maintenance fees to maintain an application in effect are listed on CIPO’s webpage on Patent Fees. Maintenance fees are due annually on or before the anniversary of the filing date, starting on the 2nd anniversary of the filing date.
Any or all of the maintenance fees for a particular application may be paid in advance. In accordance with subsection 68(3) of the Patent Rules, the time limits for payment of maintenance fees for applications cannot be extended.
Date modified: January 2024
If the full required maintenance fee is not paid on or before the anniversary date, a prescribed late fee will also need to be paid (paragraph 27.1(2)(a) of the Patent Act, and section 70 of the Patent Rules). A Commissioner’s Notice under paragraph 27.1(2)(b) of the Patent Act will be sent to the applicant shortly after the maintenance fee due date. If no payment is made before the anniversary date, the late fee is owed, regardless of whether a notice was sent. The notice will require the applicant to pay the maintenance fee and the late fee before the later of:
The period between the original due date and the later of six months from the due date or two months from the date of the notice is the late fee period. If the maintenance fee and the late fee are not paid within the late fee period, the application will be deemed abandoned under paragraph 73(1)(c) of the Patent Act. The application can be reinstated under subsection 73(3) of the Patent Act. Please see Chapter 9 for more information on abandonment and reinstatement.
Payments of the maintenance fee and the late fee, during this period, will be acknowledged to the default correspondent in a courtesy letter.
Date modified: January 2024
Divisional applications carry their own maintenance fees, separate from the parent application. Maintenance fees are calculated as a function of the filing date of the divisional (which is the same as the parent application) according to subsection 68(2) of the Patent Rules.
All maintenance fees between the filing date and the presentation date of the divisional are due on the presentation date. If they are not paid by that date, a Commissioner’s Notice under paragraph 27.1(2)(b) of the Patent Act will be sent requiring them to be paid along with a single late fee before the later of six months after the presentation date or two months after the date of the notice.
Date modified: September 2020
Applications filed under the provisions of the Patent Cooperation Treaty and entering the national phase in Canada must pay maintenance fees (see CIPO’s webpage on Patent Fees) at the date of PCT national phase entry. Note that the international filing date is the date on which the maintenance fee schedule is based.
Date modified: October 2019
All patent applications are opened to public inspection after the end of a confidentiality period under section 10 of the Patent Act. The fundamental purpose of opening to public inspection is to encourage innovation by disclosing the details of inventions to the public to encourage the distribution of information and enable other inventors to remain abreast of the latest developments in their field and to develop improvements. Public inspection is the trade-off for the time limited monopoly granted to inventors and their legal representatives by patents.
All patent applications, except those filed prior to October 1, 1989 and documents on file in connection therewith, shall be open to public inspection after the expiration of an eighteen-month confidentiality period (subsection 10(2) of the Patent Act). The end of the confidentiality period is the earlier of:
Date modified: October 2019
Applications filed under the Patent Cooperation Treaty (PCT) are also laid open to public inspection by the World Intellectual Property Organization (WIPO) eighteen months after filing or, where a request for priority has been made, eighteen months after the earliest priority date claimed. Under section 157 of the Patent Rules, if an application was published in English or French by WIPO on or before the national phase entry date in Canada, the application is considered to be open to public inspection under section 10 of the Patent Act as of the international publication date. If not, the public inspection date will be when it is laid open to the public in Canada.
Date modified: October 2019
In accordance with subsection 10(2) of the Patent Act, an applicant may make a written request to have an application opened to public inspection before the expiry of the confidentiality period. In office practice, this is commonly known as ‘Early Laid Open’. There is no fee attached to this service request.
Date modified: June 2021
Applications not yet open to public inspection are confidential under section 10 of the Patent Act and sections 16, 17 and 18 of the Patent Rules establish the conditions for access to them during this period. The Commissioner will provide access to information respecting an application for a patent that is not open to public inspection to an applicant, a patent agent appointed in respect of that application or any person authorized by the applicant (if there is a single applicant) or by the common representative (if there are joint applicants).
Authorized persons must provide identification when requesting access to a file. Persons permitted access by the applicant, common representative, or patent agent must provide identification and must furnish a signed document granting that person authorization. The signed document must contain the patent application number and contact information of the applicant, common representative, or patent agent, and must be signed by the applicant, the common representative, or patent agent. Inventors who have assigned all interest in their invention to others will not have access to an unopened file without authorization from the applicant, common representative, or patent agent. If an agent has been appointed and the inventor has retained some interest in the application, the inventor may see the file and discuss the case with the examiner in general terms but, in accordance with section 39 of the Patent Rules, an interview including a detailed discussion of the prosecution is permitted only in the agent's presence or with the agent's consent. As detailed in chapter 12.06, an examiner will not discuss matters relating to the prosecution of an application with persons other than the agent or those who have the agent's permission to discuss the application.
Date modified: October 2019
After an application is laid open to public inspection, the public can access the application and information related to it in a variety of means.
The application, the complete prosecution history and all documents filed in connection with the application or resulting patent may be viewed in person at CIPO in Gatineau, Quebec, purchased online via the Data and Document Dissemination Section, or obtained by contacting the Data and Document Dissemination Section at:
Data and Document Dissemination Section
Canadian Intellectual Property Office
Innovation, Science and Economic Development Canada
Place du Portage, Phase I
50 Victoria, Room C-229
Gatineau QC K1A 0C9
Tel.: 1 866 997-1936 (from 8.30 a.m. to 4:30 p.m. EST)
Fax: (819) 953-9969
Date modified: September 2020
The Canadian Patent Database (CPD) is an online database available 24 hrs a day, 7 days a week where applications, patents, administrative information and most documents filed in connection with applications and patents are available. Please click here for more information on protecting your privacy on the Canadian Patents Database
Date modified: October 2019
All documents relating to a patent or a patent application submitted by applicants, patentees, the public and patent agents are open to public inspection under section 10 of the Patent Act after the confidentiality period has expired. Most of that information is made available online on the Canadian Patents Database. However, the Patent Office will endeavour to prevent the posting of sensitive personal information to the Canadian Patents Database if document(s) are expressly identified by the applicant as having sensitive personal content. Therefore, it is recommended that any sensitive personal information, such as personal medical information, be provided in a separate supporting document with the submission and clearly marked as sensitive. Please click here for more information on protecting your privacy on the Canadian Patents Database.
Date modified: September 2020
On Tuesday of each week, except for statutory holidays, a list of all patents granted during the week ending on that Tuesday and a list of all patent applications that became open to public inspection during that week are published on the website of the Canadian Intellectual Property Office.
Date modified: September 2020
An application for a patent may be withdrawn at any time. A request for withdrawal must be in writing from the person authorized to represent the applicant (see Chapter 5). It may be possible to refund fees of a withdrawn application other than the application fee, for a regular Canadian application, or other fees in respect of International applications, such as transmittal, search, preliminary examination, or basic national fees, for a PCT application, if the application was filed through inadvertence, accident or mistake and it was withdrawn not later than the fourteenth day after the earliest date that the documents were submitted to the Commissioner to establish a filing date (paragraph 139(1)(b) of the Patent Rules) or entry into the national phase (paragraph 139(1)(c) of the Patent Rules).
Date modified: September 2020
Under subsection 10(5) of the Patent Act, a patent application will be removed from active files and not be made open to public inspection if the application is withdrawn on or before the earlier of the day that is two months before the end of the confidentiality period and, if applicable, the day on which a request is made for early opening to public inspection. Requests to withdraw the application after the end of confidentiality and before the issue of the patent will have the effect of removing the application from active files, but will not undo the effect of publication already in effect and public disclosure.
Date modified: October 2019
A request for priority may be withdrawn at any time before a patent is issued. If the applicant withdraws a request for priority at an early stage it may be possible to delay the opening of the application to public inspection (subsection 10(4) of the Patent Act). The withdrawal must be made within sixteen months of the filing date of the earliest priority application (section 17 of the Patent Rules). The application will then be laid open to public inspection at the end of the new confidentiality period (eighteen months from the Canadian filing or eighteen months from the earliest of any other priority date, if more than one priority was claimed).
Date modified: September 2020
A cover page is prepared by the Patent Office for all patent applications when the application is laid open to public inspection and again in preparation for grant. The cover page contains administrative or bibliographic information about the patent application or patent. The following data fields are included in the cover page:
(22) Filing Date
(45) Issue Date
(86) PCT Filing Date
(87) PCT Publication Date
(41) Date Open to Public Inspection
(30) Priority Data
(21) Number assigned to application
(12) Plain language designation of type of document
(13) Kind of document code according to WIPO standard 16
(85) National Entry Date
(86) PCT Application number
(87) PCT Publication number
(51) International patent classification
(54) Title of the invention
(71) Names of applicant(s)
(72) Names of inventor(s)
(73) Names of owner(s)
(74) Names of Agent(s) or attorney(s)
There are two distinct points in time where the Patent Office publishes the cover page during the prosecution of a patent application.
The first point in time is the laying open of the patent application to public inspection which follows the expiry of the confidentiality period that ends eighteen months after the earlier of either the filing date or the earliest priority date. In anticipation of the opening of the application to public inspection, the Patent Office will prepare a cover page for the patent application, which contains an identification of various bibliographic data. As part of this process, current versions of the abstract and claims are rendered into a searchable form of text.
The second point in time is upon the issue date of the patent grant. A new cover page will be created that will contain all the bibliographic data current up to the date of the patent issue. The versions of the abstract and claims, as they were allowed and granted, will be rendered into searchable text.
The cover page is a static document and cannot be altered under normal circumstances. Any subsequent change in the bibliographic data after publication as the result of change, recordal or correction, will not initiate a re-creation, correction, or re-publication of the cover page though it will be visible in Office records as well as online in the Canadian Patent Database.
Corrections to, or any other change to the database of the Office may result in an overnight update to the Canadian Patents Database and internal records. It may appear from time to time that bibliographic data, as indicated above and on the published cover page at the time when the application becomes open to public inspection will not reflect the instantly updated data on electronic or web-based versions of the same application.
Date modified: August 2021
The Patent Office has a practice with regards to special characters and how they are entered in Office database and records. Special characters are those beyond the standard 26 letter Roman alphabet, numerals 0 to 9 and the following characters: & (ampersand), ° (degree) and % (percentage). Characters beyond those listed above, such as letters with accents (é, è, ö, etc.) and most Greek characters are considered special characters for Office practice.
Due to technical limitations of our current database, some special characters cannot be entered in the Office records, nor can they be reliably reproduced in the Canadian Patents Database.
Consequently all special characters appearing in names, titles or any other information that is transcribed into our database will be entered into our database according to the Special Character Conversion Table presented in this Section. The converted characters will subsequently appear in bibliographic information in the Canadian Patents Database and in all correspondence originating from the Office.
Characters appearing in documents that are not transcribed into the database are not affected by this technical limitation as these documents are stored only as images in our database.
Characters appearing on the Cover Page are not affected by this technical limitation. This will result in special characters appearing on the Cover Page but not on other documents produced by the Office.
Special Characters Conversion Table
|
Special Character |
Will be Entered as |
|
æ, Æ |
A |
|
ä, Ä |
A |
|
â, Â |
A |
|
á, Á |
A |
|
á, À |
A |
|
å, Å |
A |
|
ß |
SS |
|
ç, Ç |
C |
|
é, É |
E |
|
è, È |
E |
|
ê,Ê |
E |
|
ë, Ë |
E |
|
ï, Ï |
I |
|
î, Î |
I |
|
í, Í |
I |
|
ì, Ì |
I |
|
ñ, Ñ |
N |
|
œ, Œ |
O |
|
ø, Ø |
O |
|
ö, Ö |
O |
|
ô, Ô |
O |
|
ù, Ù |
U |
|
ü, Ü |
U |
|
û, Û |
U |
|
ÿ, Ÿ |
Y |
|
«» |
`` `` |
|
Special Character (Greek Alphabet) |
Will be Entered as |
|
Α, α |
.ALPHA. |
|
Β, β |
.BETA. |
|
Γ, γ |
.GAMMA. |
|
Δ, δ |
.DELTA. |
|
Ε, ε |
.EPSILON. |
|
Ζ, ζ |
.ZETA. |
|
Η, η |
.ETA. |
|
Θ, θ |
.THETA. |
|
Ι, ι |
.IOTA. |
|
Κ, κ |
.KAPPA. |
|
Λ, λ |
.LAMDA. |
|
Μ, μ |
.MU. |
|
Ν, ν |
.NU. |
|
Ξ, ξ |
.XI. |
|
Ο, ο |
.OMICRON. |
|
Π, π |
.PI. |
|
Ρ, ρ |
.RHO. |
|
Σ, σ, ς |
.SIGMA. |
|
Τ, τ |
.TAU. |
|
Υ, υ |
.UPSILON. |
|
Φ, φ |
.PHI. |
|
Χ, χ |
.CHI. |
|
Ψ, ψ |
.PSI. |
|
Ω, ω |
.OMEGA. |
Date modified: October 2019
This chapter provides guidance on topics relating to the abandonment and reinstatement of patent applications.
Date modified: October 2022
Moving a patent application to a granted patent requires the applicant to comply with several administrative requirements (such as submitting information and documents to render the application compliant, paying annual maintenance fees, etc.) and to have it examined for compliance with the Patent Act and the Patent Rules, to ensure that it is patentable. The patent system is constructed so as to move the application from its filing date to either a patent grant or rejection in a reasonable time frame in order to limit the time when rights are uncertain in order not to have a chilling effect on third parties who may wish to use that invention.
Failure by the applicant to respond or comply with the requirements outlined in the Patent Act and the Patent Rules will result in the application being deemed abandoned under subsections 73(1) or 73(2) of the Patent Act. The list provided in section 9.02.01 lists the causes for deemed abandonment. A patent application can be subject to multiple, concurrent or overlapping abandonments.
It is the responsibility of the applicant to meet all obligations under the Patent Act and Patent Rules that are necessary to avoid the abandonment of a patent application.
If the application is abandoned after examination has started, examination will be suspended until the application is reinstated.
Date modified: October 2019
An application for patent shall be deemed to be abandoned under subsection 73(1) of the Patent Act if:
Date modified: October 2022
An application for patent will also be deemed to be abandoned under subsection 73(2) of the Patent Act in any other circumstance provided for in subsection 132(1) of the Patent Rules. Under subsection 132(1), the application will be deemed to be abandoned if:
the applicant does not make a request for continued examination of an application for a patent and pay the prescribed fee in accordance with subsection 85.1(3);
a notice of allowance is sent under subsection 86(1), (6), (10) or (12) and the applicant does not pay the final fee (see CIPO’s webpage on Patent Fees) within the time referred to in the applicable subsection;
the applicant does not reply in good faith to a conditional notice of allowance of the Commissioner sent under subsection 86(1.1) and pay the final fee (see CIPO’s webpage onPatent Fees) within the time referred to in that subsection; and
Date modified: October 2019
While not required by the Patent Act or the Patent Rules, the Patent Office will endeavour to inform applicants of deemed abandonments through a courtesy letter. Please note that in all cases, applicants will have received a notice of the potential for abandonment if they didn’t comply with the requirements.
Date modified: September 2020
Where an application is deemed to be abandoned under subsection 73(1) or 73(2) of the Patent Act, the application may be reinstated by the applicant according to subsection 73(3) of the Patent Act within 12 months of the date the application was deemed abandoned by:
Note that the 12-month time period to reinstate and the reinstatement fee are prescribed by sections 133 and 134 of the Patent Rules.
Date modified: October 2022
If an application is abandoned because the applicant failed to respond to an examiner’s requisition made by the examiner under subsection 86(2) or (5) of the Patent Rules and because they failed to request continued examination under subsection 85.1(3) of the Patent Rules, the applicant does not need to pay two fees to request reinstatement as long as both failures are mentioned in the reinstatement request.
Date modified: October 2019
Certain reinstatements also have the added requirement for a positive determination by the Commissioner that the failure occurred in spite of the due care required by the circumstances having been taken under paragraph 73(3)(b) of the Patent Act.
The following requests for reinstatement of applications deemed abandoned require a positive determination of due care (as prescribed under section 135 of the Patent Rules):
Therefore, any request of reinstatement where a determination of due care by the Commissioner is required should be accompanied by a statement of the reasons for failure which led to the abandonment.
For more information on the due care standard and how it will be applied, please see Section 9.04 in this Chapter.
Date modified: October 2019
Once a patent application is deemed to be abandoned, the applicant has 12 months to reinstate the application.
A few examples are listed below for illustrative purposes:
Example 1:
An examiner’s requisition dated January 15 requires a response within four months; therefore the time limit for a response is May 15 of the same year. A response is not provided by May 15 and therefore the application is deemed abandoned on May 15. The reinstatement period ends on May 15 of the following year.
Example 2:
The maintenance fee for an application is due on Aug 29, 30 or 31 and it is not paid by the due date. The Commissioner’s Notice is sent on September 15 requiring the applicant to pay the fee and late fee before the later of 2 months from the date of the notice or 6 months from the maintenance fee due date. The later date is 6 months from the maintenance fee due date or February 28 (or February 29 in leap years) of the following year. The maintenance fee and the late fee is not paid by February 28 (or February 29 in a leap year) and therefore the application is deemed abandoned on February 28 (or February 29 in a leap year). The reinstatement period ends on February 28 of the next year.
Example 3:
A Commissioner’s notice sent under section 65 of the Patent Rules requiring the applicant to comply within three months of the notice is sent on March 31. The applicant is required to respond by June 30. A response is not provided by the applicant by June 30 and therefore the application is deemed abandoned on June 30. The reinstatement period ends on June 30 of the following year.
Date modified: October 2019
If an application is deemed to be abandoned for multiple failures, a single request to reinstate the application may be made so long as a reinstatement fee is paid in respect of each failure and all of the actions are taken to rectify all the failures that caused the abandonments. In the case of a single request for multiple failures, the requests’ reinstatements must be made before the end of the first reinstatement period.
Date modified: September 2020
Amendments made to the Patent Act and the Patent Rules (SOR/2019-251) to implement the Patent Law Treaty (PLT) introduce a due care standard that must be met by an applicant before an application deemed abandoned can be reinstated after the following has occurred:
Subject to the transitional provisions below, the Commissioner is required to make a positive determination that a failure has occurred – in spite of the due care required by the circumstances having been taken – before the application can be successfully reinstated following the failures outlined above.
Date modified: October 2022
An applicant requesting reinstatement of an application following a failure to pay a maintenance fee or a failure to request examination that occurred prior to October 30, 2019 is not subject to the due care standard. Section 73 of the Patent Act, as it read immediately before October 30, 2019, applies with respect to such requests for reinstatement.
Date modified: October 2019
In order for the Commissioner of Patents to make a determination, the applicant is required to provide the reasons for the failure to take the action that should have been taken to avoid abandonment of the application. The application will be reinstated if the applicable requirements set out in paragraph 73(3)(a) of the Patent Act are met and if the Commissioner determines, based on the reasons provided by the applicant, that the failure occurred in spite of the due care required by the circumstances having been taken and informs the applicant of this determination.
Date modified: October 2019
When determining whether the failure occurred in spite of the due care required having been taken by applicant, the Commissioner will assess whether the applicant took all measures that a reasonably prudent applicant would have taken – given the particular set of circumstances related to the failure – to avoid the failure, and despite taking those measures, the failure occurred. Measures taken by the applicant after the failure occurred will not be taken into consideration in making the determination. This approach is generally consistent with the approach that is currently used by CIPO when acting as a Receiving Office in the context of a request for restoration of priority under the Patent Cooperation Treaty, when that request for restoration of the right of priority is made on the basis that due care required by the circumstances was taken.
Date modified: September 2020
The applicant must, within 12 months after the date the application was deemed to be abandoned, meet the following requirements set out in paragraph 73(3)(a) of the Patent Act to reinstate the application:
Date modified: September 2020
If the request for reinstatement is submitted more than six months after the prescribed time to request examination, the applicant must meet the following requirements set out in paragraph 73(3)(a) of the Patent Act to reinstate the application before the end of 12 months after the application is deemed abandoned:
Date modified: October 2019
In order to make a determination of whether the failure occurred in spite of due care required by the circumstances having been taken, the Commissioner will consider the reasons for the failure to act that are provided by the applicant. In order to assist the Commissioner in making a determination, the Patent Office recommends that the applicant include, as part of the required reasons for the failure, the following elements in the request for reinstatement:
The applicant may also include evidence of the circumstances and reasons for failure such as a medical note, or other relevant affidavits. For information on protecting your privacy, please see Section 8.02.05a in Chapter 8.
Date modified: October 2019
The Commissioner will review the reasons for the failure to pay the maintenance fee and the late fee, or the reasons for the failure to request examination in time and pay the late fee to determine whether the failure occurred in spite of the due care required by the circumstances having been taken. In making a determination of whether the failure occurred in spite of the due care required under the circumstances having been taken, the Commissioner will consider whether anything else could have been reasonably expected to have been done to avoid the failure while taking into consideration the particular set of circumstances surrounding the failure to take the required action. Measures taken by the applicant after the failure occurred will not be taken into consideration in making the determination. In making this determination, the Commissioner will consider the customary diligence that a prudent party would have exercised in the circumstances.
In making this determination, the Patent Office will have regard to considerations that are taken into account by the International Bureau and Receiving Offices as described in paragraph 166M of the Receiving Office Guidelines, while acknowledging that no two cases have identical sets of facts or circumstances.
In general, under the following circumstances, a determination that a failure occurred in spite of due care required under the circumstances having been taken by the applicant, the patent agent or other person authorized by the applicant may be made where those people demonstrate the due care of a reasonably prudent person that would be required by the circumstances was taken:
In general, the following circumstances may favour a determination that due care required by the circumstances was not taken by the applicant, the patent agent or other person authorized by the applicant:
Date modified: October 2019
Before any determination is made by the Commissioner under paragraph 73(3)(b) of the Patent Act on whether the due care required by the circumstances was not taken, the Patent Office will send a letter to the applicant or patentee informing them of the Commissioner’s intended determination and provide the applicant with the opportunity to make observations before the end of one month after the date of the letter.
Date modified: October 2022
Unless the applicant is informed that the Commissioner intends to determine that due care required by the circumstances was not taken, applicants can expect a response to a request for reinstatement, including the Commissioner’s determination with respect to the due care standard, within six months of receipt of the request in the Office, or six months from receipt of the last correspondence relating to the request.
Date modified: October 2019
Rights are afforded to third parties who, while the patent rights are uncertain, take actions in good faith that would otherwise constitute an infringement. In specific circumstances, third parties are protected against infringement proceedings when they start using or make serious and effective preparations to use a patented invention after a prescribed period of time has elapsed after patent rights appear uncertain.
Failure to pay a maintenance fee on a patent application and failure to request examination of a patent application and provide the prescribed fee could give rise to third party rights. (Section 55.11 of the Patent Act and section 128 of the Patent Rules.)
Date modified: January 2025
Fees with respect to patent applications, patents and other services related to them can be consulted on CIPO’s webpage on Patent Fees, which includes categories on domestic applications, international applications, and patents.
The Tariff of Fees set out in Schedule 2 of the Patent Rules does not reflect current fee amounts for all fees. The Patent Fees web page should be considered the official source of patent fee information.
Information relating to the extensions of time for fee payments can be found in sections 2.03.03g, 2.03.03h and 2.03.03i of this manual.
Date modified: September 2020
The World Intellectual Property Organization (WIPO) is the administrative body that oversees international IP treaties, such as the Patent Cooperation Treaty. Types of PCT fees paid during the international phase of the PCT application lifecycle, such as the transmittal fee, the search fee, and the international filing fee are payable to the receiving office. Upon entry into national phase, the basic national fee is payable to the national IP office. Consult CIPO’s web site for information on the PCT Schedule of Fees here.
Please see Chapter 34 for more information on the Patent Cooperation Treaty.
Date modified: January 2025
Fee amounts set by the Patent Office are adjusted annually, with yearly rates posted on the Patent Fees page. In respect of international applications during the international phase, some fees are collected by the Patent Office for the benefit of the World Intellectual Property Organization (WIPO). The amounts of these fees are set by WIPO, and do not follow a defined adjustment schedule.
The amount due for a given fee generally depends on the date on which the Commissioner receives the complete fee payment, not the date which the service was originally requested, nor the prescribed date to pay the fee. Exceptions exist for “top-up fees” (see 10.01.02a, 2.03.03g, 2.03.03h and 2.03.03i) and certain international application fees (see 10.01.02c).
For fees set by the Patent Office subject to an annual adjustment:
For example, if, after you have filed a Canadian application and obtained a filing date, but have not paid the application fee and a notice was sent prior to the January of the next year, the amount you should pay will depend on when you pay it. If, in response to the notice, you pay the full amount prior to January, then you must pay the prescribed amount for the current year. If you have paid on or after January 1 of the next year, then you must pay the adjusted prescribed fee for next year, despite any amount indicated in the notice.
Similarly, the amount due for a maintenance fee depends on the date on which your payment is received, not the prescribed date to pay the fee. This applies whether the fee is paid early, during the late fee period, or during the reinstatement period.
Date modified: January 2025
If a fee is paid in two or more partial payments, the amount of the fee to be paid is the amount that would be payable if the full amount of the fee had been paid on the day on which the last partial payment is received.
Exceptions exist for partial payments in the form of certain authorized ‘top-up’ fees, for which an extension of time must be granted. For information to identify the payment amounts for these ‘top-up’ fees, please consult 2.03.03g, 2.03.03h and 2.03.03i of this Manual.
Date modified: January 2025
The amount of the fee to be paid for an examination of a patent application is:
If the person requesting examination does not pay a sufficient amount to cover the excess claims in the application, and the applicant subsequently amends the application to attempt to cause the request for examination to become compliant (see 11.01.01 and section 83.1 of the Patent Rules), they should be aware that the total examination fee due is the amount owing on the date of the amendment, which may differ from the amount that was owing at the time of the original payment (subsection 5.02(3) of the Patent Rules).
Date modified: January 2025
In contrast to the amount due guidance detailed in section 10.01.02 of this manual, the fee amount for international applications is the amount payable as of the date of receipt of the international application for the following fees:
Date modified: September 2020
Certain fees are reduced for small entities such as small business and universities to encourage them to use the patent system. To pay fees at the small entity amount, an applicant or patentee must:
Date modified: January 2024
The Patent Rules defines a small entity as one that employs fewer than 100 employees or that is a university. This does not include:
Note: small entity status is determined at the filing date or the national phase entry date of the patent application. If a company employed 10 people at the filing date or the national phase entry date of and then grows to 200 people five years later, it still qualifies as a small entity.
Date modified: October 2022
Where an applicant or patentee wishes to pay small entity fees, a small entity declaration must be submitted. A signed small entity declaration can be included in the petition or can be submitted as a separate document. The small entity declaration can be signed by:
Additionally, the name of the applicant or patentee and, if applicable, the name of the patent agent or registered foreign practitioner signing the declaration must be indicated. The following small entity declaration tool is available to applicants and patentees.
A fee may only be paid at the small entity rate in respect of an application or a patent if a signed small entity declaration is submitted by the deadline applicable for that fee. If a standard fee payment was made prior to the compliant small entity declaration and also before the deadline applicable for that fee, the applicable fee is the prescribed standard fee. If a small entity declaration is submitted after a standard fee is paid, but before the fee-payment deadline, the standard fee payment will not be considered an overpayment and a refund of the difference between the standard and small fee amounts will not be provided for that particular payment.
Date modified: December 2020
For many administrative steps in the lifecycle of an application for a patent, such as filing, requesting examination, and maintaining the application, the payment of a fee will be a requirement. The client must provide the necessary financial information with every payment, in which the general correspondence rules must also be observed when communicating with the Office (see Chapter 2 on Communications).
To better facilitate payment from clients, CIPO allows a variety of methods of payment (see Section 10.03.01 in this Chapter) and has developed a Fee Form for user convenience (see Section 10.03.02 in this Chapter).
Please see the practice notice on the Fee Payment Practice of CIPO, effective since June 8, 2009 for more information. PN – CIPO Fee Payment Practice.
Date modified: September 2020
Payments to CIPO for all requisite fees for Patent Office business must be made in Canadian dollars. In addition to traditional payment methods by cheque or credit card, fees can also be paid electronically via CIPO’s electronic payment service, which first requires obtaining an activation code.
Consult the CIPO web site for more information on methods of payment. CIPO – methods of payment
Date modified: October 2019
As an additional method of payment, CIPO offers the option of opening a Deposit Account, whereby the client may load funds and whereby the Office may freely withdraw funds upon specific client request to fulfill patent lifecycle requirements.
Detailed instructions to open, replenish and utilize deposit accounts can be consulted on CIPO’s website. CIPO – Deposit Accounts.
Date modified: December 2020
Whether a payment is made by mail, fax, or in person, it is strongly recommended to use the Fee Form developed by CIPO. This will better facilitate the processing of payment, while better securing client financial information. Although the Fee Form should accompany written communications concerning fees, it will not be made a part of the patent application file open to public inspection, nor will the form be viewable with the file contents on the Canadian Patent Database. CIPO regards the security of the client’s financial information to be of primary importance.
Information concerning the form can be consulted on the CIPO website here.
Note that online electronic payments can be done via the Office’s e-services/e-payment tools. This can be viewed on the CIPO website here.
Date modified: September 2020
In limited situations where a payment deficiency or shortfall has occurred, recourse may be taken in general authorization statements included in timely client communications instructing the charge of the balance or the entirety of fees where there was a clear and obvious intent to pay. Such statements do not exempt the client from undertaking other necessary actions or fulfilling their responsibility to maintain their applications in good standing. That responsibility cannot be transferred to the office.
These statements cannot be employed to cover speculative or conditional situations beyond the client’s clearly stated intention in the specific context of the correspondence in which the authorization appears. Nor can such statements be used to act as an automated authority to manage other aspects of a file outside of the immediate context. For example, an applicant submits a letter whose sole purpose was to request examination and pay the associated fee. No fees are included with the letter. A maintenance fee is also due on the day the letter is submitted to the Office and no explicit instructions to pay the maintenance fee were present in the document. However, the letter does contain a general authorization statement asking the Office to debit all missing fees from a deposit account as well as any other fees that are due and to reinstate the application if it is abandoned. In this particular circumstance, the Office will debit the missing request for examination fee from the deposit account since there was an explicit instruction to pay this fee in the letter. The Office would not have debited the maintenance fee since there was no explicit instruction to pay the maintenance fee in the letter which occurred beyond the situation that the letter was expressly written to address. Furthermore, since the maintenance fee was not charged, any conditional reinstatement request could not have been acted on since the complete action that would have had to have been undertaken in order for the reinstatement to be effective was not done.
The practice notice, effective since June 8, 2009, on the use of General Authorization to charge a deficiency can be consulted on the CIPO website here.
Date modified: January 2025
The date an individual or bulk payment is received by the Commissioner is the date of the physical delivery to the Patent Office or the date of receipt at the local time of the Patent Office, if delivered by electronic means, as the case may be (see 2.03.01).
Where a fee is paid in partial payments, for the purpose of establishing when a fee was paid with respect to its prescribed date, the date that the Patent Office received the complete fee is used. For example, where payments received by the Office either failed or were insufficient with respect to the amount prescribed under the Rules, the date of the initial insufficient or attempted and failed payment is not used if that fee is eventually paid in full.
Exceptions exist for deemed dates of payment for ‘top-up’ fees (see 2.03.03g, 2.03.03h, 2.03.03i) and the date of payment for a partial request for examination (see 10.04.01).
Date modified: January 2025
In the case of a payment for the fee for the request for examination, where an initial payment was insufficient to cover the excess claims fee portion and where an amendment subsequent to the insufficient payment reduces the number of claims such that the payment of the fee for the request for examination is no longer insufficient (see 11.01.01 and 10.01.02b), then the amount paid is considered to have been received on the day on which the amendment was made.
Date modified: December 2025
The Commissioner will issue refunds following a written request by the client if permitted by subsection 139(1), of the Patent Rules. The refundable fee types or refund conditions are as follows:
In accordance with subsection 139(2) of the Patent Rules, the Commissioner does not issue refunds under paragraphs 139(1)(b) to (h) if the request for a refund is received later than three years after the day on which the fee was paid.
Maintenance fees for a particular application or patent that are paid in advance are not eligible to be refunded, with the exception of those eligible for a refund under paragraph 139(1)(b) of the Patent Rules.
Any fee, or any part of a fee, that is required to be remitted under the Service Fee Act is not eligible for refund (subsection 139(3) of the Patent Rules).
Date modified: October 2022
The Commissioner may waive payment of the fees for the request of a correction under subsection 109(1) of the Patent Rules, to submit a request to reissue a patent under section 47 of the Patent Act, or to apply for an extension of time when there is a delay in receipt of an examiner’s report (see section 2.03.03(e)(ii)).
Date modified: October 2022
In order to waive the fee for requesting a correction or to submit a request for reissue of a patent, the correction or reissue request must be the result of an error by the Commissioner and the Commissioner must be satisfied that the circumstances justify the waiver.
The Office encourages patentees to make the request for the waiver of the fee with their request for a correction or reissue as well as provide a justification explaining how the error resulted from the Commissioner. The Office will review the request for a waiver and the justification. If the fee has been provided and the Commissioner agrees to waive the fee, it will be automatically refunded without request. If the fee has not been provided and the Commissioner does not agree to waive the fee, then the fee will be requested before the Office proceeds any further with the request for a correction or a reissue.
Date modified: October 2022
In order to waive the fee for requesting an extension of time in the case of delayed receipt of an examiner’s report, the Commissioner must be satisfied that the circumstances justify the waiver.
In general, the fee will be waived if the following requirements are met:
Under these circumstances, the Commissioner may extend the period of time to reply to the examiner’s requisition to a maximum of 6 month from the date of receipt of the examiner’s requisition (see section 2.03.03e(ii)).
If the above requirements to waive the fee are met but the applicant does not provide any justification for an extension of time, the Commissioner will extend the time to reply to the examiner’s requisition to 4 months from the date of receipt of the examiner’s requisition.
If the applicant requests an extension of time beyond 4 months from the date of receipt, the applicant must provide justification for the extension and pay the fee for requesting an extension of time as set out in section 2.03.03e. The fee will not be waived.
The Office encourages applicants to make the request for the waiver of the fee with their request for an extension of time. The Office will review the request for a waiver. If the fee has been provided and the Commissioner agrees to waive the fee, it will be automatically refunded without request. If the fee has not been provided and the Commissioner does not agree to waive the fee, then the fee will be requested before the Office proceeds with the review of the request for an extension of time.
Date modified: December 2025
In cases where an applicant or patentee relied on erroneous payment information provided by the Commissioner, the Commissioner may waive payment of the difference between the amount that was paid and the amount of the fee that should have been paid on the day on which the insufficient payment that was required under subsection 3(4) of the Patent Rules. To waive payment the Commissioner must:
In effect, this ‘top-up’ would not require any action to be taken on the part of the applicant or patentee.
Date modified: December 2025
In cases where the Commissioner waived payment based on erroneous information as described above, the Patent Rules allow for a transition period of 4 months in which applicants or patentees can benefit from an extension of time without taking action, in cases where the same erroneous information was provided, the period of time for payment of the fee has not expired, and the Commissioner considers that the circumstances justify the waiver (subsection 139.1(2) of the Patent Rules).
Date modified: October 2022
CIPO is committed to delivering administrative services in the most efficient manner possible without compromising the high quality and timeliness that clients have come to expect. Service standards reflect our current levels of service, however in the practical sense, this means delivering the best service in the least time, subject to our operational capabilities, resources and infrastructure.
With the advent of the Service Fee Act, given Royal Assent on June 22, 2017, we aim to meet CIPO’s service standard targets 100% of the time.
Consult CIPO’s web site for information on client service standards here. For more information about remissions under the Service Fees Act, see CIPO’s web page on Remissions at CIPO.
Date modified: October 2019
CIPO is committed to providing service to its clients according to defined performance targets, and regularly measures whether or not these commitments are being met. Measuring performance and monitoring progress are important elements of planning. These processes allow us to assess what we have achieved over time and determine where there is room for the organization to improve.
Consult CIPO’s web site for information on performance targets here.
Date modified: October 2022
Under Canada’s patent system, a patent application is not examined automatically when it is filed. Canada operates on a system of deferred examination, wherein an application is only examined upon request. This Chapter provides guidance on the administrative procedures and practices that are necessary when requesting the examination of a patent application and the types of requests. For more information on substantive examination practices see Chapters 12 to 23 in this manual.
In accordance with subsection 35(1) of the Patent Act, a request for examination may be made by any person*, as long as it is in the manner specified in section 79 of the Patent Rules and accompanied by the necessary fee prescribed in subsection 80(1) of the Patent Rules (see CIPO’s webpage on Patent Fees). The prescribed fee for requesting examination is composed of the basic fee and a fee for each claim in excess of 20 included in the application (see section 11.01.01). There are reduced fee amounts for the basic fee if the application has been the subject of an International Search by the Commissioner, under the PCT. The Commissioner of Patents also has the authority under subsection 35(2) of the Patent Act to require an applicant to make a request for examination of an application.* If a request for examination for a patent is made by a third party, the Patent Office will inform the applicant of this fact.
In accordance with section 79 of the Patent Rules, the following information must be provided with any request for examination:
Date modified: January 2025
The prescribed fee for requesting examination is composed of the basic fee and a fee for each claim in excess of 20 included in the application (see CIPO’s webpage on Patent Fees). When a request for examination is made the person making the request will be responsible for counting the number of claims in the application and paying the appropriate fee. If more than 20 claims are included, the person requesting examination must pay the excess claim fee for each claim in excess of 20 in order for the Office to consider the request for examination to be successfully made. The Office will verify that the correct amount has been paid by counting the number of claims contained in the application on the date that the request for examination is made.
A claim that defines the subject-matter of an invention in the alternative, including a dependent claim within the meaning of section 63 of the Patent Rules that refers to more than one preceding claim, counts as a single claim for the purposes of determining the number of claims.
If the person making the request did not pay the proper fee, including the excess claim fee, the request for examination is not successfully made. The person making the request can pay additional monies so the proper fee is paid or the applicant can amend the application after requesting examination to reduce the number of claims such that the amount that was paid is greater than or equal to the amount due. The amount paid for requesting examination will be considered paid on the day the additional monies are paid or the amendment is made, as the case may be.
If the number of excess claims is increased after requesting examination and the patent application is in condition for allowance, the applicant will be notified in the notice of allowance of the requirement to pay for the increase in excess claims as part of the final fee. The excess claim fee amount due as part of the final fee will be assessed based on the maximum number of claims in the application at any time after the request for examination is made until the day the final fee is paid (see sections 25.01 and 25.01.01).
Date modified: October 2019
The time limit to request examination under subsection 35(2) of the Patent Act is prescribed under subsection 81(1) of the Patent Rules. The request must be made before:
Date modified: January 2024
If a request for examination is not received within the prescribed time limit a late fee will be required in addition to the examination fee (s35(3)(a) of the Patent Act, and section 82 of the Patent Rules) irrespective of any mailing of a Commissioner’s Notice. The Commissioner’s Notice under paragraph 35(3)(b) of the Patent Act will be sent to the applicant shortly after the due date. The notice will require the applicant to make the request and pay the request for examination fee, including the excess claim fee owing (see section 11.01.01) and the late fee before the end of two months after the date of the notice.
The period between the original due date and two months from the date of the notice is the late fee period. If the request is not made and the request for examination fee and the late fee are not paid within the late fee period, the application will be deemed abandoned under paragraph 73(1)(d) of the Patent Act. The application can be reinstated under subsection 73(3) of the Patent Act. Please see Chapter 9 for more information on abandonment and reinstatement.
Date modified: October 2022
Once formalities processing is completed and a request for examination is made under section 35 of the Patent Act, the patent application is referred to the appropriate patent examiner and is examined in due course. For examination timelines see CIPO’s Service Standards here.
Date modified: October 2019
Patent applications are generally examined sequentially according to the date on which the request for examination was made. There are, however, mechanisms by which the examination of a patent application may be advanced out of routine order. These mechanisms include “special order” advanced examination; advanced examination of applications related to green technology; and the Patent Prosecution Highway.
Date modified: January 2025
Under paragraph 84(1)(a) of the Patent Rules, the Commissioner of Patents shall advance an application for examination out of its routine order on the request of any person who pays the fee (see CIPO’s webpage on Patent Fees) and who files with the Commissioner a statement that failure to advance the application is likely to prejudice that person’s rights. The filing of the statement itself is an administrative requirement, so the Commissioner makes no determination on the veracity or appropriateness of the statement, nor has the discretionary authority to refuse a request under paragraph 84(1)(a) of the Patent Rules on the basis of a determination of the veracity or appropriateness of the statement as long as that statement has been given clearly in the initial request. Applications that are subject to advanced examination are commonly referred to as “special order” examinations.
In accordance with subsection 84(1) of the Patent Rules, a “special order” request for advanced examination can only be granted if the application in question is open to public inspection under section 10 of the Patent Act and if a request for examination has been made in compliance with the Patent Rules. Please see Chapter 4 for information on compliance requirements.
Once a patent application is advanced out of its routine order, this generally applies for the duration of its prosecution; however, subsection 84(2) of the Patent Rules specifies that an application will be returned to its routine order if:
A person who requested the “special order” examination can also request that the advanced examination cease, in which case the application will be examined in its routine order. The fee for requesting an advanced examination is not refundable.
Date modified: September 2020
Under paragraph 84(1)(b) of the Patent Rules, examination of a patent application relating to green technology can be advanced out of routine order upon request. The applicant must submit a statement stating that the application relates to technology “the commercialization of which would help to resolve or mitigate environmental impacts or conserve the natural environment and natural resources.” The filing of the statement itself is an administrative requirement, so, the Commissioner makes no determination on its veracity or appropriateness, nor has the discretionary authority to refuse a request under paragraph 84(1)(b) of the Patent Rules on the basis of a determination of the veracity or appropriateness of the statement as long as that statement has been given clearly in the initial request. No additional fee is required.
In accordance with paragraph 84(1)(b) of the Patent Rules, a request for advanced examination of an application related to green technology can only be granted if the application in question is open to public inspection under section 10 of the Patent Act, and a request for examination has been made in compliance with the Patent Rules. Please see Chapter 4 for additional information on compliance requirements.
Date modified: September 2020
The Patent Prosecution Highway (PPH) allows an applicant to significantly accelerate the examination of their patent application, provided that they have a corresponding application deemed allowable by one of Canada’s international PPH partners.
In order for a patent application to qualify for the PPH, a request must be received before examination has begun, all claims must sufficiently correspond to one or more of the claims found allowable by the partner Office and the application must already be open to public inspection. No additional fee is required to pursue PPH.
Date modified: October 2022
The purpose of examination is, at each stage, to perform a thorough analysis of the patent application to determine whether it complies with the requirements of the Patent Act and the Patent Rules. After a request for examination is made, an examiner will analyze the application taking into consideration the originally filed application and any amendments that have been subsequently received in the Patent Office.
Date modified: October 2022
In the initial stage of examination, after examining the application for defects with respect to the Patent Act and Patent Rules, the examiner will:
Where an examiner’s report is issued, the applicant has four months from the date of the report to respond with arguments and/or amendments that cause the application to comply with the Patent Act and Patent Rules. See sections 11.05.03 and 11.05.05 for more information on submitting an amendment in response to an examiner’s report.
It is possible to request an extension of time to the prescribed time to respond to the examiner’s requisition up to six months after the date of the requisition under subsection 3(1) and section 131 of the Patent Rules. Please see section 2.03.03 for more information on extensions of time.
Date modified: October 2022
After the applicant responds to an examiner’s report, the examiner will take into account any arguments and amendments and assess whether the application complies with the Patent Act and Patent Rules. Similar to the initial stage of examination, this may result in the examiner issuing: a notice of allowance; a conditional notice of allowance; an examiner’s report; or an interview (see 11.04.01). However, unlike in the initial stage of examination, the examiner in later stages of examination may reject the application in a specific type of examiner’s report called a Final Action. For more information on the rejection of an application and Final Action reports, see Chapter 26.
Subject to the issuance of a notice of allowance or a conditional notice of allowance is issued, after a request for examination is made, the examiner will send a maximum of three examiner’s reports detailing defects in the application[26]. In order for examination to continue after the third report is sent, or after a notice of allowance or a conditional notice of allowance is issued, a request for continued examination will be required (see section 11.04.02a).
Date modified: October 2022
If, after the initial request for examination is made, the examiner has issued three examiner’s reports detailing the application’s defects, the applicant will be required under subsection 85.1(1) of the Patent Rules to request continued examination in order for examination to continue after the third report is sent.[27]
If, following the notification of the need to request continued examination, the request for continued examination is not made on time, the application will be deemed abandoned under paragraph 132(1)(e) of the Patent Rules.
In the course of examination, if a notice of allowance or a conditional notice of allowance is issued before three examiner’s reports detailing the application’s defects are sent, the applicant will be required to request continued examination under subsection 85.1(4) of the Patent Rules in order for examination to continue after the notice of allowance or a conditional notice of allowance is issued.
Examiner’s reports that have been withdrawn do not count towards the number of reports sent for the purpose of requesting continued examination (see 12.04.03 for more information on withdrawal of an examiner’s report).
Date modified: October 2022
If a compliant request for continued examination is made, the examiner may send up to a maximum of two more reports. In order for examination to continue after the second report is sent after a request for continued examination is made, a subsequent request for continued examination under subsection 85.1(2) of the Patent Rules will be required.[28]
The examiner will notify the applicant in the second report they must request continued examination and pay the prescribed fee not later than four months after the date the notice is sent. The applicant will be required to request continued examination under subsection 85.1(2) of the Patent Rules in order for examination to continue after they have been notified.[29]
If, following the notification of the need to request continued examination, the request for examination is not made on time, the application will be deemed abandoned under paragraph 132(1)(e) of the Patent Rules.
Date modified: December 2025
An applicant can also request continued examination under subsection 85.1(4) of the Patent Rules after a notice of allowance or a conditional notice of allowance is sent. A compliant request will set aside the notice of allowance or conditional notice of allowance. The applicant must make the request and pay the prescribed fee (see CIPO’s webpage on Patent Fees) no later than four months after it was sent and before the day on which the final fee is paid. If a compliant request for continued examination is made, the examiner may send up to a maximum of two more reports (see section 11.04.02b). After a compliant request for continued examination is made, 85.1(1) of the Patent Rules does not apply and a notice requiring a request for continued examination will be sent by the examiner only under 85.1(2) of the Patent Rules, i.e. if two examiner’s reports have been sent since the most recent request for continued examination was made.
It is important to note that applicants may not request continued examination to set aside the notice of allowance or conditional notice of allowance under subsection 85.1(4) of the Patent Rules when the application has been deemed abandoned for failure to pay the final fee under subsection 73(2) of the Patent Act and paragraph 132(1)(f) or 132(1)(g) of the Patent Rules.
In accordance with subsection 100(3) of the Patent Rules, if a conditional notice of allowance is set aside, any amendments made to the application from the day the conditional notice of allowance is sent until the day on which the conditional notice allowance is set aside are considered never to have been made.
Date modified: October 2022
Once a filing date has been established, the applicant can amend the content of their application, the specification (which consists of the description and the claims) and the drawings before the patent is issued (subsection 38.2(1) of the Patent Act). Once a notice of allowance or a conditional notice of allowance is issued, no amendments are permitted, other than what can be narrowly defined as an obvious error under section 100 of the Patent Rules, unless the amendments are those referred to in a conditional notice of allowance or the notice of allowance or conditional notice of allowance are withdrawn or set aside. The amendment is made to the application on the day it is submitted.
Applicants can submit an amendment voluntarily or in response to a requisition or a conditional notice of allowance. The Office will process any request by the applicant to amend a patent application. However, those amendments must also abide the overarching restriction defined in subsection 38.2(2) of the Patent Act, wherein any such amendment must not add new matter that cannot reasonably be inferred from that which was contained in the application at filing (see Chapter 20 for more information on new matter). Amendments causing changes to the number of claims may have an effect on the excess claim fee required when requesting examination or paying the final fee (see section 25.01 and 25.01.01).
The request must be made in writing and comply with the requirements outlined in the Patent Rules. Please see section 2.02 for information on requirements for submitting written communications to the Office and Chapter 5 for information on who can represent applicants with respect to prosecution of patent applications.
Amendments submitted by those not authorized to represent the applicants will be placed as a document on file, but not entered as an amendment. In other words, the content of the application visible in the ‘Claims’, ‘Description’ and/or ‘Representative Drawing’ tabs on the Canadian Patents Database will not be updated. The document will be visible in the ‘Documents’ tab.
Date modified: October 2022
A voluntary amendment may be made to a patent application at any time during the prosecution and before the issuing of the notice of allowance or a conditional notice of allowance; however they will not be entered nor examined until a request for examination has been received. In other words, the document will be visible in the ‘Documents’ tab on the Canadian Patents Database when it is received by the Office, but the amendment will not be visible in the ‘Claims’, ‘Description’ and/or ‘Representative Drawing’ tab until a request for examination is received.
A voluntary amendment will be considered to be publicly disclosed on the later of the date the application is laid open to public inspection or on the date the amendment is placed on file. Note that this could have implications for the patentability of any new matter disclosed in the amendments, particularly if there is desire to file a new patent application based on the disclosed new matter. Please see sections 18.01.01a, 18.02.01a, 18.04 and Chapter 20 for more information.
Date modified: October 2022
Amendments made to Patent Cooperation Treaty (PCT) applications during the international phase under Article 19 and 34 made before the national phase entry date in Canada become part of the national phase application at the time of national entry. Whether those amendments under Articles 19 and 34 were submitted by the applicant or otherwise made available from WIPO’s Patentscope database, the specification and drawings on file as of national entry will incorporate those pages, replacing each page altered by that amendment. Otherwise, any amendments made during the international phase after the national phase entry date in Canada will not be included in the PCT national phase application as amendments under PCT articles. However, after the national phase entry date, the applicant may submit any amendment voluntarily, subject to the new matter requirements described in Chapter 20.
Date modified: October 2022
During the course of examination, the examiner may identify defects in the specification or drawings of an application, and communicate these defects to the applicant with an examiner’s report (see 11.04.01, 11.04.02 and 12.04), or in a notice, such as a conditional notice of allowance (see 25.01.01) or a notice requiring additional drawings (see 12.05.01).
When an amendment to the specification or drawings is submitted in response to an examiner’s report or notice, a written statement (see section 11.05.05a) must explain the manner in which the amendment overcomes the defects.
Date modified: January 2025
An applicant may submit a claim amendment in error, and this error could impact the excess claim fee owing as part of the final fee (i.e. because the amendment includes an increased number of claims), should the application proceed to allowance (see 25.01). Under subsection 87(1.2) of the Patent Rules, it is possible, in some circumstances, to have the amendment deemed not to be included in the application for the purposes of final fee determination.
In order to be eligible to have the claim amendment deemed not included for final fee determination, the following criteria must be satisfied:
Date modified: October 2022
The applicant may submit a correction to the translated specification or drawings of a PCT national phase application. It may be necessary to correct a translation, rather than submit an amendment where issues of new matter could arise (see Chapter 20).
Date modified: October 2019
All amendments to the specification and drawings must be made by submitting a new page to replace each page altered by the amendment. It must also be accompanied by a statement explaining the purpose of the amendment and identifying the differences between the new pages and the replaced pages as outlined in section 102 of the Patent Rules.
Date modified: October 2022
Each amendment must be submitted with a cover letter that includes a statement of purpose noted in the preceding paragraph as well as instructions for entering the amendment. The statement and set of instructions should be suitable to the given amendment and may be considered, in its context, within a broad range of what is compliant, be it a simple or implicit instruction to replace a page or a more detailed explanation with the inclusion of a “blackline” version of the text.
Amendments are done by the submissions of new pages to replace those being modified by the amendment.
Under the rules for written communications, an amendment will be deemed to be received on the date that the Office receives the physical or electronic delivery of the correspondence. However, during the course of the substantive examination if the examiner cannot determine what is intended to be changed in a proposed amendment after considering the covering page including the statement and instructions, then the replacement pages may be removed from the specification and/or drawings as the amendment does not comply with section 102 of the Patent Rules. In such cases, where the amendment is submitted in response to an examiner’s report or notice (see 11.05.03), the submission will be considered a response to the requisitions or requirements contained within the report or notice, despite the removal of the replacement pages from the specification and/or drawings. This may cause the prosecution of the patent application to be needlessly prolonged. The issue of a non-compliant statement or instructions may be addressed in a subsequent examiner’s report or interview.
Subsection 50(1) of the Patent Rules requires the pages of the specification to be numbered consecutively. Page numbering which includes letters is acceptable; for example the sequence 1, 2, 3, 3A, 3B, 4 is acceptable. If pages are deleted, the applicant should renumber the affected pages to ensure that pages are numbered consecutively. Alternatively, the applicant may insert a numbered blank page in place of a deleted page as long as the blank page is marked with a diagonal stroke or a “Z” to indicate that no text is missing and that the space is intended to be left blank. Likewise for deletions which have resulted in partially blank pages, the applicant may insert a “Z” or a diagonal stroke to fill areas of empty space to indicate that no text is missing and the space is intended to be left blank. Section 61 of the Patent Rules requires the claims to be numbered consecutively in Arabic numerals beginning with the number 1.
The instructions should clearly indicate how the replacement of those pages should be made.
All submissions of amendments must comply with the requirements outlined in the Patent Rules to submit written communications to the Commissioner. Please see Chapter 2 for more information.
Date modified: October 2022
The cover letter should follow the following format and order:
Date modified: September 2020
There are no regulatory requirements governing the manner in which the text matter in an item of written communication to the office is organized or formatted, other than to abide by subsection 8(1) of the Patent Rules which stipulates that, with exceptions, those communications must relate to one patent or one application at a time.
The Office can make only informal recommendations on this subject in view of fostering best practices. The applicant should consider reasonable steps in the presentation, a clear indication of each service or action request, and a sensible grouping of topics in their communications to better facilitate efficient processing and to reduce operational risk of mishandling.
If items are sent by physical mail, it should be noted that those items may not be received by the Office in the order in which they were initially dispatched by the sender. Further, incoming correspondence is manually sorted on an item-by-item basis, therefore service requests on discrete pieces of correspondence on a related application or patent number may be treated in the order in which they were processed and may not necessarily be treated in the order that the applicant or patentee intends. If the applicant chooses to include these multiple matters in a single submission pertaining to an application or patent, the applicant should clearly indicate these requests on the first page of the cover letter. Some examples are listed here:
Where an amendment submission also includes a PPH request form, this should also be mentioned in the cover letter.
Date modified: January 2025
Under subsection 86(17) of the Patent Rules, the examination of patent applications is suspended in the following situations:
While examination is suspended, applicants will not typically receive reports or notices from the examiner; however, if any notices are sent or received during a period of suspension, the notices are considered valid.
Date modified: January 2025
As introduced in Chapter 11, the purpose of examination is, at each stage, to perform a thorough analysis of the patent application to determine whether it complies with the requirements of the Patent Act and Patent Rules. As part of this thorough analysis, the examiner performs a purposive construction of the claims (see 12.02), and considers a search of the prior art (see 12.03).
After examining the application, if the application is considered compliant with the Patent Act and Patent Rules, the examiner will approve the application for allowance, generally resulting in a notice of allowance in accordance with subsection 86(1) of the Patent Rules (see chapter 25).
Where the examiner considers that the application does not comply with the Patent Act and Patent Rules, they may communicate the defects to the applicant using:
Note that an application for which examination has been requested may be examined prior to being laid open to public inspection under section 10 of the Patent Act, but an examiner will not approve an application for allowance, nor issue a conditional notice of allowance, until it has been laid open.
Date modified: April 2018
A detailed discussion regarding examination of the abstract, description and drawings can be found in chapters 13, 14 and 15 of this manual, respectively.
Date modified: October 2022
In Canada (Attorney General) v Amazon.com Inc, the Federal Court of Appeal observed that, during examination, Supreme Court jurisprudence “requires the Commissioner’s identification of the actual invention to be grounded in a purposive construction of the patent claims”
.[30]
The application of the principles of purposive construction to the examination of a patent application must take into account the role of the patent examiner and the purpose and context of examination.[31]
In Free World Trust and Whirlpool, the Supreme Court outlined that purposive construction is performed by the court to objectively determine what the person skilled in the art would, as of the date of publication of the patent application and on the basis of the particular words or phrases used in the claim, have understood the applicant to have intended to be the scope of protection sought for the disclosed invention.[32]
It should be noted that, in accordance with subsection 15(3.2) and 155.5(4) of the Patent Rules, text matter that is not in English or French:
should not be taken into account for the purposes of interpreting the scope of protection sought or obtained for the invention, unless that text matter is in free text of a sequence listing, in a non-language dependent field.[33]
Once a claim has been purposively construed, that construction is used to determine whether the claim complies with the Patent Act and Patent Rules. Where there is no disagreement as to the construction of a claim, the examiner may choose not to provide the detailed purposive construction analysis in a report.
Date modified: June 2015
When examining a claim, an examiner must read the claim in an informed and purposive way. Prior to construing a claim an examiner will:
The above steps provide the context in which the claim is to be read. Once the context is determined the examiner will:
Date modified: June 2015
Claim construction during examination therefore requires an examiner to interpret each claim in a structured manner whereby the examiner will:
Date modified: June 2015
The specification as a whole is addressed to the person skilled in the art and, as such, provides the context in which the claim should be read and informs the meaning of the terms recited in the claim and the nature of the invention.[34] Upon a purposive construction the terms of a claim will be given specific technical meanings in light of the common general knowledge of the person skilled in the art.[35] Thus, in order to arrive at a fair, balanced and informed understanding of the subject-matter of a claim, it is critical that a purposive construction of the claim be performed considering the specification as a whole as read through the eyes of the person skilled in the art, against the background of the common general knowledge in the field or fields relevant to the invention at the time the application became available to the public.
During examination, the necessary foundation of knowledge for performing a purposive construction of the claims is found in submissions from the applicant and the knowledge of an appropriately experienced examiner.[36]
Date modified: October 2019
As detailed above, prior to construing the claims, the examiner must first identify the person skilled in the art.
The person skilled in the art (POSITA) is a fictitious construct that represents an average worker competent in the field or fields relevant to the invention.[37] The person skilled in the art can represent an individual, or a team of individuals whose conjoint knowledge is relevant to the invention in suit.[38]
The person skilled in the art is considered to be competent; a logical but unimaginative worker in the field,[39] who is neither a dull-witted incompetent nor a creative, intuitive expert.[40] In a highly technical field, the person skilled in the art may be presumed to have expert-level knowledge and skills.[41] The skilled person need not be a manufacturer or designer, but must understand the problem to be overcome, have knowledge of means to address the problem and the likely effect of using the means.[42] Furthermore, the person skilled in the art is reasonably diligent in keeping up with advances in the field or fields of relevance to the invention,[43] and has the advantage of being multilingual and thereby being able to comprehend prior art in any language.[44] Note that the person skilled in the art may have knowledge from outside the field of the invention, although it should not be presumed that they would.[45] In this context, the nature of the problem being addressed by the alleged invention may be helpful in defining the skilled person.
During examination, the person skilled in the art is relevant in many contexts. It is important to recognise that there is only a single description of the person skilled in the art for a given alleged invention. Nevertheless, the common general knowledge of the person skilled in the art will depend on the date at which an understanding of the application is required.[46] Note that in some circumstances, this can require the person skilled in the art to rely on knowledge which, while generally accepted at the relevant date, was later shown to be wrong.[47]
Depending on the specifics of a given case, it may be necessary to explicitly identify the person skilled in the art. It should be stressed that this is not necessary where the nature of the person skilled in the art does not appear to be under debate or where it is unlikely to impact on any conclusions as to patentability.
Where the specific nature of the person skilled in the art is relevant for resolving an issue during examination, the examiner will determine who this person is by reference to the field or fields relevant to the invention and will consider comments by the applicant in the determination. The person skilled in the art may be ascertained from the language of the specification of the application.[48] Attributes such as proclivity for engaging in research or experimentation may help form the profile of the skilled person.
Although the characterisation of the person skilled in the art should be done carefully,[49] it should also be done with a certain degree of generalisation.[50] During examination, an examiner must attempt to interpret the application using the appropriate knowledge that the person skilled in the art would have possessed at the relevant date. Specific details such as the skilled person’s exact educational background or length of work experience are typically unnecessary and have the potential to be misleading or overly restrictive; precise definitions of the skilled person should therefore be avoided.
Date modified: October 2019
After identifying the person skilled in the art (see
12.02.02b), the examiner must identify
the relevant common general knowledge (CGK) of the person of ordinary skill in the art at relevant date. The relevant date for construing the claims is the publication date.
“Common general knowledge means knowledge generally known by persons skilled in the relevant art at the relevant time.”
[51] The common general knowledge in a field has been described as the knowledge that emerges as common themes from the “forest of art”, and which becomes commonly known to the ordinary person skilled in the art.[52] This knowledge undergoes continuous evolution and growth.[53]
The common general knowledge distinguishes the body of information that is widely recognised from that which is simply publicly available. Individual disclosures may become common general knowledge, but only when they are generally known and regarded as a good basis for further action.[54] At the same time, some information that forms part of the common general knowledge may not have been written down at all.
Where the common general knowledge in a field becomes relevant for the purposes of examination, examiners may refer to information they believe to have been common general knowledge as of the relevant date. Unless it becomes evident through the applicant’s comments that the nature of the common general knowledge is not common ground and is reasonably in dispute, an examiner need not identify documents establishing the common general knowledge.
Where it is appropriate or necessary to establish the common general knowledge in a field (for example where the examiner and applicant disagree as to the common general knowledge), this can be done by citing established reference works (such as textbooks, review articles, handbooks, etc.) or by demonstrating commonality of certain knowledge in a number of disclosures in the field. The common general knowledge at a certain date can be confirmed by subsequent publications,[55] or by showing that the knowledge had been accepted in the field over a period of time. Statements in the application’s description that describe certain information or knowledge as commonly known may be relied upon, without verification, as establishing aspects of the common general knowledge; an applicant will be bound to such comments.[56]
Date modified: October 2019
The purpose of the Patent Act is to provide exclusive rights to an inventor for a new and useful invention in exchange for a disclosure that allows the public to use or operate the invention as contemplated by the inventor. Thus, recognizing that a patentable invention is an inventive solution to a practical problem[57], it follows that an invention must be disclosed (and ultimately claimed) so as to provide the person skilled in the art with an operable solution.
The identification of the problem and the solution provided by the invention informs the purposive construction of the claims.[58]
The identification of the problem faced by the inventor is guided by the examiner’s understanding of the common general knowledge in the art and by the teachings of the description.
The common general knowledge in the art provides the baseline of information to which the description is expected to add. The person skilled in the art will read the specification in the expectation that it sets out something beyond the commonly known solutions to commonly known problems.
It must be borne in mind that the applicant is not required to explicitly state the problem and solution. Paragraph 56(1)(d) of the Patent Rules makes this clear, stating:
a description of the invention must be set out in terms that permit the technical problem and its solution to be understood, even if that problem is not expressly stated.
Consequently, the identification of the problem and its solution may be an integrated exercise, i.e. the manner in which the solution is described can help inform the problem, and vice versa. For example, a significant focus in the description on certain details of the solution may assist in the identification of the problem, while a relative absence of emphasis on certain aspects of the solution may likewise suggest the problem lay elsewhere. Where the applicant is explicit as to the nature of the problem, examination should generally proceed accordingly unless doing so would be unreasonable on an informed reading of the application in light of the common general knowledge.
The examiner will give consideration to what the inventor states about the background of the invention, the “objects of the invention”, any specific problems, needs, limitations or disadvantages known in the art or discovered by the inventor, etc. in identifying the problem faced by the inventor.
While claim construction during examination must remain anchored in the language of the claims, it “cannot be determined solely on the basis of a literal reading” of the claims.[59] A properly informed purposive construction must consider the application as a whole.
Not only must one not lose sight of the fact that the claims must be interpreted in light of the description, a claim-based analysis “does not mean that the Commissioner cannot ask or determine what the inventor has actually invented, or what the inventor claims to have invented. On the contrary, these are relevant and necessary questions in a number of contexts, including novelty, obviousness, and patentable subject-matter”
.[60] This is consistent with the recognition in Free World Trust of the need to avoid “the pitfalls of language” so as to ensure the inventor receives “protection for that which he has actually in good faith invented”.[61]
Date modified: June 2015
One aspect of purposive construction is the identification of the essential elements of the claim. The identification of the essential elements of a claim cannot be performed without having first properly identified the proposed solution to the disclosed problem. Without having first considered the problem and solution, the identification of essential elements would be circular – it would begin and end with the language of the claim, contrary to Free World Trust which recognizes that elements can be found to be non-essential if at the date of publication of the patent, the skilled addressee would have appreciated that a particular element could be substituted or omitted without affecting the working of the invention.[62]
Ultimately, some element or combination of elements defined in the claim must provide the solution. One must, however, approach each claim with an understanding that not every element that has a material effect on the operation of a given embodiment is necessarily essential to the solution. Some elements of a claim define the context or the environment of a specific working embodiment, but do not actually change the nature of the solution to the problem.[63] .
Note that while the identification of the essential elements is performed in light of the common general knowledge of the art at the date of the publication of the patent specification,[64] this does not mean that one can simply conclude that the essential elements of the invention are those that distinguish the claimed subject-matter from the prior art.[65] That is, an element is not necessarily essential merely by the fact that is not found in the prior art. Likewise, an element cannot necessarily be deemed non-essential merely because it is part of the CGK. An element is essential if it is required to provide the solution to the problem, regardless of whether or not it is known.
Having identified the problem and solution, and defined the essential elements in the claims, an examiner may conclude that the claim either omits an essential element or includes non-essential elements.
Where it appears, having considered a claim in light of a fair reading of the description, that an element essential to the operation of the solution has not been defined in the claim, the claim may be defective for over breadth (i.e. lack of support) and/or for lack of utility.
In certain cases, an examiner may consider elements included in a claim of an application to be superfluous (non-essential) to the solution to a given problem. The mere presence of a superfluous limitation is not a defect as such, although the inclusion of such an element could render a claim defective (for example if its presence results in ambiguity).
It must be recognized that while the Office considers superfluous elements to be non-essential and not relevant to the determination of a claim’s patentability during examination, if an applicant maintains such an element in the claim through to grant a court might later construe it to be essential when applying the “self-inflicted wound” factors of purposive construction as identified in Free World Trust and Whirlpool.[66]
An invention is an element or a combination of elements that provides a solution to a problem. Where a claim includes solutions to more than one problem it includes more than one invention.[67]
If a claim includes solutions to more than one problem, examination should focus on one solution to a problem in performing the purposive construction. The initial choice of solution should be guided by the description, selecting the solution given the greatest emphasis by the inventors. If it becomes necessary to consider a different solution, the analysis should be undertaken anew.
On occasion it may be the case that elements or sets of elements in a claim do not interact with each other to achieve a unitary result; this may reflect an “aggregation” rather than a combination.[68] A consideration of the problem and solution emphasized by the inventor in the description may assist the examiner to select only the element or set of elements that work together in the claim that provide the operable solution.
Date modified: June 2015
In most cases, an examiner reading a claim will automatically ascribe appropriate meanings to the terms of a claim in light of the teachings of the description and the examiner’s technical expertise. It is not necessary to explain these conclusions in a report, unless it becomes apparent that there is some relevant disagreement between the examiner and the applicant as to the significance of certain terms. In such instances, it is only necessary to explicitly address the construction of the contested terms.
Similarly, in some cases it will be possible to conclude that a claim does not comply with the Patent Act or Patent Rules without explicitly determining whether a given element is or is not essential. A prior art document that discloses all the elements of a claim, for example, will anticipate the claimed subject-matter regardless of whether each element is essential or not. Here again, examiners are not required to detail in reports parts of their analysis that are not in issue.
Where an examiner’s conclusions regarding a specific element are relevant to the identification of a perceived defect, the examiner should provide reasons to support their conclusions, e.g. emphasize the identified problem and solution and those elements essential to providing that solution.
Once the claims have been purposively construed, the essential elements can be analyzed to determine if they clearly define subject-matter that complies with the Patent Act and Patent Rules. Specific requirements are discussed in the following chapters of the MOPOP:
Requirements for the clarity and form of the claims are covered in Chapter 16.
Subject matter is covered in Chapter 17.
Utility is covered in Chapter 19.
Novelty, obviousness and double patenting are covered in Chapter 18.
Date modified: June 2015
The following examples apply the guidance set out in this section to a determination of statutory subject matter.
Example 1:
An application is directed to a known skillet and a known spoon, where the skillet and spoon each incorporate a “specific identifier” in the form of a feature common to both products. The description indicates that it is known in the art to provide silicone grips on spoon handles to improve a user’s grip on a spoon, and that it is known to include a silicone grip on a skillet handle to help insulate the handle. The application discloses that by incorporating the specific identifier in the handles of both products, a consumer is likely to buy the two products together due to the recognition of the specific identifier. The description indicates that the specific identifier has aesthetic appeal and that embodiments include a raised logo molded into the silicone and a specific striped pattern.
Claim:
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is considered to be a person who is skilled in the design, production and manufacturing of cookware including the fields of metal working, forging, and plastic moulding. The POSITA is knowledgeable in the field of marketing.
Common general knowledge (CGK)
The description states that the use of silicone on spoon handles to improve the grip is CGK and that the use of silicone on skillet handles is widely known as an insulator. Though an examiner could independently verify that the use of silicone on cookware is CGK, if an applicant explicitly states that certain knowledge is CGK the examiner may take such statements at face value. Methods of moulding silicone grips on cookware are well known. Methods of moulding logos and various patterns in silicone are common general knowledge.
The Problem
It is clear from the description that the problem the inventor has set out to solve is to influence a consumer to associate one product (the spoon) with another product (the skillet). Considering the statements made in the description and the common general knowledge, improving the grip on the spoon and insulating the skillet handle were not part of the problem that the inventor set out to solve.
The Solution
Though the applicant has claimed the silicone-wrapped handles and refers to the respective benefits that they confer to the spoon and skillet, these benefits are not material to solving the problem of leading a consumer to associate the two products together. The solution to the problem the applicant has set out is the provision of the specific identifier on both products.
What are the essential elements?
As the specific identifiers are the only elements of the claim that provide the solution to the problem outlined in the description, the specific identifiers are the only essential elements of the claim.
Is the claim statutory?
The specific identifiers are features having a purely intellectual or aesthetic significance which do not affect the practical functioning of the products. The examiner will therefore identify a defect under section 2 of the Patent Act since the only essential elements of the claim are the specific identifiers; the claims therefore do not define a statutory invention [see chapter 17 of the MOPOP for a discussion of statutory subject-matter].
Example 2
An application is directed to a portable playpen for outdoor use. The description states that such playpens having no legs and flexible undersides are well known for use on slightly uneven terrain, such as in a park, as the flexible underside can conform to the terrain. The application discloses that the inventors set out to improve these playpens by adding a feature that would determine whether or not the playpen installation is stable and alert a parent if the installation is not stable. They have added several sensors at particularly chosen locations about the center of the playpen to gather data to calculate a stability factor. If the stability factor is below a predetermined threshold, an alarm attached to the playpen will sound.
Claim:
1. A method of determining the instability of an outdoor playpen comprising:
• providing a playpen with sensors adhered to positions X, Y and Z;
• measuring the vertical and horizontal load at each sensor;
• calculating an overall stability factor for the playpen using the data collected by the sensors; and
• sounding an alarm if the stability factor is below a predetermined threshold.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is knowledgeable in the field of children’s furniture design, production and manufacturing, and in the fields of force measurement and electronics.
Common General Knowledge (CGK)
Outdoor playpens having flexible bottoms to allow them to conform to the contours of uneven terrain are well known. It is also known that various types of sensors can be incorporated into products to calculate various values.
The Problem
It is clear from the description that the problem the inventors wanted to solve was determining whether or not an outdoor playpen having a flexible bottom is stable when placed on uneven terrain.
The Solution
The solution as detailed in the description is to adhere sensors to locations X, Y and Z of the playpen, measure force data at each sensor, calculate a stability factor from the data, and sound an alarm when the stability factor is below a predetermined threshold.
What are the essential elements?
In order to solve the problem of determining whether or not an outdoor playpen is stable, the following elements of the claim are considered essential: adhering sensors to locations X, Y and Z of a playpen, measuring the force at each location, calculating the stability factor for the playpen using the data collected by the sensors, and sounding an alarm if the calculated stability factor is below a predetermined threshold.
Is the claim statutory?
Yes. The essential elements of adhering the sensors at locations X, Y and Z, measuring the force at each location and sounding the alarm are statutory elements that have a practical application.
Example 3, Scenario a):
An application is directed to a new board game in which game pieces are moved by players around the spaces on a 3-dimensional board depending on the number resulting from a roll of a dice. The board has a mechanized arm with a claw at one end that rotates around the board; depending on the orientation of the claw and the position of the piece, the claw will either knock over the player’s piece or pick it up and place it in a different area of the board.
Claim:
A board game comprising:
a 3-dimensional game board comprising a pattern of spaces;
the board comprising a mechanized arm that is rotated around the centre of the board by a motor, said arm comprising a claw that can be positioned in two orientations, either horizontal or vertical; and
a plurality of game pieces, wherein each piece comprises a means to interact with the claw when in the horizontal orientation thereby allowing said claw to pick up said piece.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is a designer and manufacturer of board games and is also knowledgeable in the field of mechanical engineering.
Common General Knowledge (CGK)
Board games in general are well known and the manufacture of 3-dimensional game pieces is CGK. Mechanical devices are also CGK.
The problem
The problem that has been identified in the description is the need to create a new board game.
The solution:
The solution as detailed in the description is the provision of a new board game that requires a plurality of game pieces and a 3-dimensional board comprising a mechanized arm and claw.
What are the essential elements?
The elements that are required to obtain the solution are the pieces and the 3-dimensional board comprising the mechanized arm and claw.
Is the claim statutory?
Yes. The essential elements, (the 3-dimensional board, the game pieces, and the mechanized arm and claw) provide a practical solution to the problem.
Example 3, Scenario b):
Ten years after the introduction of the board game of Example 3, Scenario a) into the market, the game has achieved commercial success and is well known world-wide. The inventor has filed a new application for an improved board game that now has additional instructions printed on the spaces of the board (e.g. move ahead three spaces, back two spaces, etc…). The improved board still comprises the original mechanized arm and claw.
Claim:
A board game comprising:
a 3-dimensional game board comprising a pattern of spaces;
said board comprising a mechanized arm that is rotated around the centre of the board by a motor, said arm comprising a claw that can be positioned in two orientations, either horizontal or vertical;
and a plurality of game pieces, wherein each piece comprises a means to interact with the claw when in the horizontal orientation thereby allowing said claw to pick up said piece;
wherein 20% of the spaces comprise instructions on where to move a particular game piece.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is the same as that of Example 3, Scenario a) above.
Common General Knowledge (CGK)
Board games are CGK. The particular 3-dimensional board, pieces, mechanized arm and claw disclosed in the application are all CGK. The use of instructions on the spaces of a board game is CGK.
The problem
As detailed in the description, the problem is defined as finding an improved method of playing the game.
The solution:
The instructions printed onto 20% of the squares provide the solution to the identified problem.
What are the essential elements?
While the claim defines the game board, the pieces, and the mechanized arm and claw, these merely provide the context of the invention. They do not change the nature of the solution to the problem. The element that is essential to solve the identified problem is the inclusion of instructions on 20% of the spaces on the board.
Is the claim statutory?
No, the essential element is the inclusion of instructions that are printed on the spaces. The instructions are mere printed matter, which is not patentable subject matter.
Example 3 Scenario c):
Ten years after the introduction of the board game of Example 3, Scenario a) into the market, the game has achieved commercial success and is well known world-wide. The inventor has filed a new patent application for an improved game board having small hydraulic pistons at each corner of the board. The pistons are used to elevate and lower each corner of the board during the game so that the 3-dimensional characteristics change (i.e. the board is tilted) resulting in a changing interaction between the claw and the game pieces.
Claim:
A board game comprising:
a 3-dimensional game board comprising a pattern of spaces;
the board comprising a mechanized arm that is rotated around the centre of the board by a motor said arm comprising a claw that can be positioned in two orientations, either horizontal or vertical;
said board comprising a hydraulic piston at each corner to elevate or lower the corners thereby tilting the board;
and a plurality of game pieces, wherein each piece comprises a means to interact with the claw when in the horizontal orientation thereby allowing said claw to pick up said piece.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is the same as that of Example 3, Scenario a) above.
Common General Knowledge (CGK)
Board games are CGK. The particular 3-dimensional board, pieces, mechanized arm and claw disclosed in the application are all CGK. Hydraulic pistons per se are CGK.
The problem
The problem outlined in the description is defined as finding an improved method of playing the game.
The solution
The solution to the problem is the inclusion of the hydraulic pistons at each corner of the game board so that the board can be tilted during game play.
What are the essential elements?
The board, pieces, arm and claw provide the context for the solution to the problem but are not essential elements that lead to the solution contemplated by the inventor. The essential elements are the hydraulic pistons which allow the tilting of the game board and which can cause the interaction between the claw and the game pieces to change.
Is the claim statutory?
Yes, the essential elements (the hydraulic pistons) provide a new practical application to the game board.
Date modified: October 2022
Patentability must be assessed in view of the prior art, and it is therefore necessary for the relevant prior art to be identified. The prior art, broadly speaking, includes all information, in any form, made available to the public in Canada or elsewhere prior to the claim date[69], with a limited exception for information disclosed by the applicant or those obtaining information from the applicant (see paragraph 28.2(1)(a) of the Patent Act). In practice, however, the prior art relied on during examination generally comprises published patent documents, journal articles, textbooks, manuals and the like.
An application for patent in Canada may result from a national filing or from entry into the national phase of an international application filed in Canada or elsewhere under the Patent Cooperation Treaty (PCT).
The scope of the search of the prior art performed by a Canadian examiner at the national phase is determined in part by the extent to which relevant prior art has been identified in any earlier searches[70]. Further, examiners are not required to search claimed matter having defects relating to unity of invention (see chapter 21), divisional requirements (see section 21.10), or new matter (see chapter 20).
Where the claimed subject-matter has been the subject of a comprehensive international search by an International Searching Authority, a Canadian examiner will nevertheless perform at least a search of Canadian patent documents to identify documents relevant to double-patenting or to anticipation under paragraphs 28.2(1)(c) and 28.2(1)(d) of the Patent Act (see sections 18.06, 18.01.01c and 18.01.01d).
An examiner will typically consider available foreign search results to avoid unnecessary replication of work. Where the results of a foreign search are relied on in a report, the report should indicate which documents were identified in a foreign search.
Whenever the examiner deems it appropriate, a further search may be undertaken. This search need not be restricted to Canadian patent documents, and can be performed on any database or other search tool to which the examiner has access. Searches are generally limited by some combination of dates, keywords, and International Patent Classification (IPC) codes of relevance to the claimed matter.
In keeping with the purpose of an examiner’s report, it is desirable for all relevant prior art to be identified at the time of the first report. Nevertheless, given the sheer quantity of prior art now available it must be acknowledged that in practice documents may be missed, or that at the early stages of examination the relevance of some documents may not be fully appreciated. It is also possible that, in view of amendments to the claims or arguments presented by the applicant, it becomes necessary to rely on additional prior art.
Where, for any reason, relevant prior art is identified during the course of prosecution, it is incumbent on the examiner to cite this prior art against the claimed invention.
Date modified: October 2022
An examiner’s report is an official means of communicating with an applicant. The term “examiner’s report” or “report” means a notice from the examiner that identifies defects in the application and which contains a requisition under subsection 86(2) or 86(5) of the Patent Rules.
Under subsection 86(2) of the Patent Rules, where an examiner has reasonable grounds to believe that an application does not comply with the Patent Act and Patent Rules, the applicant must be informed of the application’s defects and must be requisitioned to amend the application to comply or to provide arguments as to why the application does comply. Where an examiner has identified one or more defects, these will be detailed in the report and, for the purposes of paragraph 73(1)(a) of the Patent Act, they are considered to be a single requisition. The beginning of this requisition can generally be identified in a report by text such as “The examiner has identified the following defects in the application”. The requisition ends with a paragraph such as “In view of the foregoing defects, the applicant is requisitioned, under subsection 86(2) of the Patent Rules, to amend the application in order to comply with the Patent Act and the Patent Rules or to provide arguments as to why the application does comply”.
In later stages of prosecution, if it appears that prosecution has reached an impasse, a specific type of examiner’s report, called a Final Action, may be issued. A Final Action includes a requisition to which the applicant must respond under subsection 86(5) of the Patent Rules. See chapter 26 for further details.
Additional requisitions under sections 85 and 94 of the Patent Rules may be included in examiner’s reports. More information on these types of requisitions is given in sections 12.04.01 and 12.04.02.
In addition to requisitions, an examiner’s report may include informative statements requiring action from the applicant. In particular, in accordance with section 85.1 of the Patent Rules, the report may inform the applicant that there is a requirement to make a request for continued examination and pay a fee in order for examination to continue. More information on the request for continued examination is provided in sections 11.04.02a and 11.04.02b.
Each separate requisition, and any separate requirement to make a request for continued examination, made in a report must be responded to within the time period indicated in the report or the application will be abandoned in accordance with paragraph 73(1)(a) of the Patent Act or 132(1)(e) of the Patent Rules. For more information on abandonment, time limits and reinstatement, see Chapter 9 of this manual.
An examiner’s report will usually include additional content that does not form part of a requisition, but which provides useful information regarding the report. This content may indicate the date of the most recent amendments and, in the case of a first report, their origin (international stage or national stage), an indication of the number of claims on file, a statement regarding the search performed, and an identification of any prior art documents discovered and a discussion of their pertinence. The report may also include general comments on the prosecution and discussions relating to points raised by the applicant in their correspondence. Where there appears to be a disagreement between the applicant and the examiner as to the construction of the claims, the report may include the examiner’s identification of the person skilled in the art and the common general knowledge. The report may also set out the examiner’s understanding of the problem that the inventor set out to solve, the solution that the inventor has contemplated, and the essential elements that lead to that solution.
An examiner’s report should be as comprehensive as possible, to enable the applicant to make informed decisions regarding the continued prosecution of their application and, if possible, to place the application in a condition for allowance [see Chapter 25]. However, there may be circumstances where a comprehensive search and examination is not conducted (see 12.03). Where an examiner has deferred the search and examination of the claims this will be indicated in the report along with the reasons leading to the deferral (see 12.03).
The examiner does not issue a report when there are reasonable grounds to believe that:
In these cases, the examiner will approve the application for allowance, or issue a conditional notice of allowance. See chapter 25 for further details.
Date modified: October 2019
Section 85 of the Patent Rules provides that where an examiner “has reasonable grounds to believe that an application for a patent disclosing the same invention has been filed, in or for any country other than Canada, by an inventor of that invention or a person claiming through them”
, the examiner may by notice requisition the applicant to provide any of the following information, a copy of any related document and/or a translation into English or French of all or part of any related document not in one of those languages:
Per section 85 of the Patent Rules, an applicant must respond to such a requisition by providing the information or document requested or by specifically stating that the information or document is not available to them.
Examiners should not requisition an identification of prior art cited in published search reports to which the examiner has ready access. Such search reports include the International Search Report, and any European Patent Office or United States Patent and Trademark Office search reports available through the respective web sites of those offices. Similarly, examiners should not requisition any information that is available to them through the web sites of those offices, including particulars of examination, opposition, or similar proceedings.
Recognising that translating documents may place a significant financial burden on the applicant, requisitions for translations should be limited to cases where no viable alternative exists.
Where a foreign language document appears relevant to examination, an examiner should attempt to locate a version of that document (or minimally of its abstract) in an Official language with which they can work. In this regard, examiners should make use of reliable online translation engines, such as that provided by the JPO, at least at the early stages of examination.
Where an examiner is working from a machine translation or from a family member of a citable document, this should be clearly stated in the report. An applicant wishing to rebut arguments made on the basis of such a document, however, may be required to provide a translation of the document to support their arguments.
Where a translation is requisitioned, the applicant must provide a translation of the document, or a part of the document, into English or French or an indication that such a translation is not available. Where only a part of the document is necessary for examination, an examiner should indicate, wherever possible, in respect of which part or parts of the document the requisition for a translation is being made.
Under Article 42 of the Patent Cooperation Treaty (PCT), no national office having received an international preliminary examination report “may require that the applicant furnish copies, or information on the contents, of any papers connected with the examination relating to the same international application in any other elected Office”
. Article 42 of the PCT applies in respect of any national phase application that has been the subject of International Preliminary Examination under Chapter II of the PCT.
The Office considers that a requisition for the identification of prior art under paragraph 85(1)(a)(i) of the Patent Rules or for application numbers, filing dates, and/or patent numbers under paragraph 85(1)(a)(ii) of the Patent Rules complies with the requirements of Article 42 of the PCT as the information being requisitioned is connected with the search of the prior art, and is not considered to be a request for copies of papers, or information on the contents of papers, “connected with examination”.
Similarly, the Office does not consider opposition, re-examination, impeachment and similar proceedings to be “connected with examination” in the sense intended by Article 42 of the PCT, and consequently requisitions under section 85 of the Patent Rules relating to such proceedings are also considered to be consistent with Article 42 of the PCT.
Date modified: October 2019
Requisitions under section 94 of the Patent Rules pertain to the inclusion in the description of the date of deposit of biological material. An examiner may requisition the applicant to amend the description to specify the date of deposit. This subject is covered in detail in chapter 23.
Date modified: October 2022
An examiner’s report may be withdrawn in exceptional circumstances where the facts of the case demonstrate a need to withdraw the report. Such situations can include, but are not limited to:
If an applicant believes that an examiner’s report should be withdrawn, a written request to withdraw the examiner’s report must be made to the Patent Office (requests made by phone will not be considered). In respect of situations defined in items iii, iv, and v, the time limit to request withdrawal of a report is the earlier of four months after the date of the examiner’s report (i.e. the due date to respond to the requisition) and the day the applicant responds to the examiner’s report. There is no time limit to request withdrawal of an examiner’s report sent in error.
If the applicant chooses to request an examiner’s report be withdrawn, the applicant should clearly denote this request in uppercase characters on the first page of the cover letter along with the date of the examiner’s report and must specify the reason(s) they believe the report should be withdrawn. For example:
Applicant requests withdrawal of examiner’s report dated [DATE]. It appears the examiner’s report is missing pages 2 and 3.
Once a request is received in the Patent Office, the reason(s) for requesting withdrawal of the report and the timeliness of the request will be evaluated and the applicant will be informed by letter whether or not the report has been withdrawn.
If a request is successful, the Office will withdraw the report. An effect of withdrawing the report is the removal of the applicant’s obligation to respond to any requisitions or requirements contained therein within the specified time limits. Another effect of withdrawing the report is that the withdrawn report does not contribute to the overall report count as it relates to the requirement to request for continued examination (see 11.04.02a). In many cases following withdrawal of a report, the Office issues a new report to which the applicant must respond. The time limit to respond to the new report is based on the date identified in the new report.
Where the request to withdraw the report relates to an allegedly improper:
the report, including the requisition under 86(2) or 86(5) of the Patent Rules, will generally not be withdrawn. Instead, if it is determined that the requisition or requirement was inappropriately included in the report, the applicant will be notified that only the specific improperly-identified requisition or requirement has been withdrawn. The withdrawal of a specific requisition or requirement in such a manner will result in the removal of the applicant’s obligation to respond to the relevant requisition or requirement without removing the obligation to respond to the other requisitions or any requirements.
Date modified: December 2020
Where an examiner’s report contains minor errors, for example, correctness and completeness of arguments in relation to noted defect(s), or typographical errors in the indication of pages , in the number of claims examined, in a defect noted, in reference to parts of the Patent Act or Patent Rules, or in prior art citations, the report will not be withdrawn. If the correction of the minor error is not immediately obvious, it is recommended that applicants raise the issue with the examiner through an applicant-initiated interview (see 12.06.01) such that any correction or clarification may be sought and be fully and clearly documented in an interview record. The applicant may also wish to include discussion on the minor error in the response to the report.
Date modified: October 2022
Date modified: October 2019
The Commissioner may see fit to require further drawings if those on file do not clearly show all parts of the invention. These may be requested by notice under section 27(5.2) of the Patent Act. If a good faith reply attempting to provide the requested drawings is not received within three months of the request date, the application is deemed to be abandoned.
Date modified: October 2022
For PCT applications that were internationally filed with some or all of the text matter in the specification or drawings in a language other than English or French, a translation of that text matter into English or French is required by the national phase entry date in accordance with paragraph 154(1)(b), 154(1)(b.1) or section 155.1 of the Patent Rules (see Chapter 34).
If, during the course of examination, the Commissioner or the examiner has reasonable grounds to believe that the translation submitted to meet the requirements of 154(1)(b), 154(1)(b.1) or 155.1 of the Patent Rules contains errors, they will issue a notice to inform the applicant of the errors. Any corrections in response to the notice must be received prior to the issuance of a notice of allowance or a conditional notice of allowance, unless the notice of allowance or conditional notice of allowance is withdrawn or set aside (see section 11.05.04). A failure to respond to the request to the corrected translation could have implications on the analysis of new matter, should any amendments attempt to rely on the erroneous content (see chapter 20).
Date modified: October 2019
During the course of examination, if consideration of a priority document based partly or entirely in a language other than English or French is necessary, an examiner may, by notice, request under section 76(1) of the Patent Rules that the applicant provide an English or French translation of part or all of said document. If the examiner has reasonable grounds to believe that such a translation is inaccurate, a certified translation may be requested under section 76(2) of the Patent Rules. Both of these notices require a response within four months, failing which, the associated request for priority will be considered to have been withdrawn (see section 7.05 for more information).
Date modified: September 2020
With respect to an application for which a request for priority on an application filed other than in Canada was made before October 30th, 2019, an examiner may, by notice, require the applicant under section 196(1) of the Patent Rules to provide within four months:
The request for priority will be considered to have been withdrawn if the request is not met, unless the applicant requests restoration of priority and provides evidence of having submitted the request to the filing office. In this case, said requested documents must be provided to the Commissioner no later than three months after the date of receipt by the applicant (see section 7.04 for more information on requests for priority and section 18.03 for more information on priority during examination).
Date modified: October 2022
Certain examiner’s reports should include a statement informing the applicant of their need to request for continued examination under section 85.1 of the Patent Rules (see sections 11.04.02a, 11.04.02b and 12.04). If a report fails to inform an applicant of their need to make a request continued examination, and that information should have been present in the report, the examiner will issue a separate notice informing the applicant of their need to make a request for continued examination.
In order to avoid abandonment under paragraph 132(e) of the Patent Rules, the applicant must request for continued examination and pay the prescribed fee no later than 4 months after the date of the notice (as described in 11.04.02a and 11.04.02b).
Date modified: June 2021
In some cases interviews may take place between examiners and the appointed patent agent, common representative or single applicant, or a registered foreign practitioner authorized by the single applicant or common representative. The ‘appointed patent agent’ may refer to either the appointed patent agent or the appointed associate patent agent. If all of the patent agents at a particular firm have been appointed as either the patent agent or the associate patent agent, any of the agents at that firm may have the interview, or give permission to a registered foreign practitioner to have the interview, as outlined below. For more information about registered foreign practitioners, please refer to chapter 5.02.02 of this Manual.
Where a patent agent has been appointed, an interview may be conducted with the common representative or single applicant or a registered foreign practitioner authorized by the single applicant or common representative only if the agent is present in the interview; or, has provided written permission for the common representative or single applicant and/or the registered foreign practitioner to conduct an interview in the agent’s absence. For a registered foreign practitioner to participate in an interview, there must always be a document on file authorizing that registered foreign practitioner to have the interview, signed by the single applicant or common representative as applicable. This requirement is additional to the permission of the appointed patent agent.
If no patent agent is appointed and there is no requirement to appoint a patent agent, only the single applicant or the common representative may have an interview with an examiner (they may not authorize anyone else to do so) (section 39 of the Patent Rules).
Interviews with examiners will be documented in the Canadian Patent Database.
Date modified: October 2022
Subject to the conditions imposed by section 39 of the Patent Rules, the appointed patent agent, common representative or single applicant or registered foreign practitioner may request an interview with an examiner in respect of an application. Appointments must be arranged in advance so that the examiner will be available and prepared to discuss the prosecution of the application. Where an agent has been appointed, the agent must be present at the interview or have authorized it.
An interview concerning the prosecution of an application, including an application that has received a final action, may be requested at any stage of the prosecution, unless the application is abandoned. The interview will be conducted by the examiner in charge of the application. During the interview the examiner may provide further explanation about the defects identified in a report or clarify certain points concerning the invention. It should be noted that interviews do not replace the normal prosecution of an application. An examiner will not provide definitive verbal opinions or agree to accept amendments to the specifications during an interview.
In the case of an interview with a new examiner in training, a senior examiner or a section head will also be in attendance. Problems that do not concern the examination process are referred to the appropriate section of the Patent Office.
The Commissioner does not meet with applicants or agents about prosecution issues related to specific applications.
Date modified: October 2022
The Patent Examination Interview Service promotes direct communication between CIPO's patent examiners and patent agents or unrepresented inventors by allowing for the prosecution of patent applications by telephone, or using an online platform for video- or tele-conferencing. The Service encourages patent examiners to contact the appointed patent agent or common representative or single applicant if no agent has been appointed by phone in situations where advancing prosecution is likely, such as when there are only a few minor defects remaining in an application.
The Service offers the appointed patent agent (or common representative or single applicant if no agent has been appointed) an opportunity to discuss the application directly with the examiner, obtain suggestions or advice from the examiner as to how an identified defect may be overcome, and correct any identified defects through written submission of a voluntary amendment within a predetermined timeframe. Any voluntary amendments submitted as a result of a phone interview are reviewed by the examiner expeditiously and the application is approved for allowance, if the amended application complies with the Patent Act and Patent Rules.
The predetermined timeframe above does not have any effect on the standing of the application; if a voluntary amendment is not received by the end of the predetermined timeframe, the examiner will simply issue a report based on the last received amendments.
Date modified: October 2019
Pursuant to section 55 of the Patent Rules, an application must contain an abstract. An abstract is not a requirement for obtaining a filing date; however, an abstract is required for the patent application to be compliant. The abstract must be in English or French and in the same language as the rest of the application (section 46 of the Patent Rules). At grant the Office translates the abstract into the other official language to better enable searching in both official languages.
Section 55 of the Patent Rules sets forth the required form and content of the abstract and requires that the abstract:
Section 49 of the Patent Rules specifies that the abstract shall commence on a new page separate from the description, the drawings and the claims. For clarity, it should have a separate heading, such as, "Abstract of the Specification". Since the abstract will be used as a search tool, the text should avoid patent jargon so that it may be readily understood by technicians and scientists and other persons who are interested in obtaining information about laid open patent applications and issued patents. It should provide a means for quickly determining the subject-matter of the specification so that the reader can decide whether a more detailed review of the document is warranted. The abstract should not refer to purported merits or speculative applications of the invention, and should not compare the invention with the prior art.
The abstract shall not contain drawings, however it may contain chemical or mathematical formulae (Section 51 of the Patent Rules).
Date modified: October 2019
A feature mentioned in the abstract and illustrated by a drawing in the application may be followed by a reference character referred to in a drawing, placed between parentheses (subsection 55(6) of the Patent Rules). In the field of biotechnology, the identifier of a sequence listing, such as “SEQ ID NO:1" may be used in the abstract to refer to the sequence listing.
Date modified: October 2019
Abstracts are subject to examination in respect to their conformance with section 55 of the Patent Rules. In addition to setting forth the form and content of the abstract, subsection 55(8) of the Patent Rules states that the abstract “must not be taken into account for the purpose of interpreting the scope of protection sought or obtained.”
Following an amendment to the specification and drawings, the abstract cannot form the basis of support for subject-matter that was not present or reasonably inferred from the specification and drawings as originally filed.
During examination, under subsection 55(7) of the Patent Rules the Commissioner is authorized to amend or replace a non-compliant abstract. In general a non-compliant abstract will be identified as a defect in a requisition under subsection 86(2) of the Patent Rules; however, in the event that a non-compliant abstract is all that prevents allowance of an application, the Examiner will directly amend or replace the abstract prior to approving the application for allowance rather than identifying such a defect in a further report.
Date modified: September 2014
The following examples illustrate what are considered to be suitable abstracts.
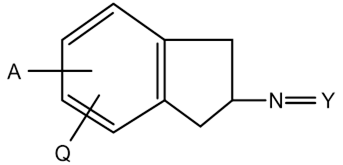
wherein A and Q are hydrogen or alkoxy groups and Y means an alkylene group with 4 to 7 carbon atoms, are useful as plant desiccants.
Date modified: October 2019
The description, together with the claims, form the specification of an application.[71]
Although the claims play a prominent role in the patent system, in that they define the scope of the exclusive privilege conferred by a patent, a proper description is fundamental to a valid patent. As was noted by the Supreme Court, “[d]isclosure is the quid pro quo for valuable proprietary rights to exclusivity which are entirely the statutory creature of the Patent Act”
[72].
The present chapter discusses the various requirements for proper disclosure under subsection 27(3) of the Patent Act as well as the various requirements as to the form and content of a description under the Patent Rules.
Date modified: December 2010
The description must provide a clear and complete disclosure of the invention such that the person skilled in the art:
In Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd., Dickson J. noted that “the inventor must, in return for the grant of a patent, give to the public an adequate description of the invention with sufficiently complete and accurate details as will enable a workman, skilled in the art to which the invention relates, to construct or use that invention when the period of the monopoly has expired”
.[74] The description must be able to answer the questions “What is your invention?: How does it work?”
[75] such that “when the period of the monopoly has expired the public will be able, having only the specification, to make the same successful use of the invention as the inventor could at the time of his application”
.[76]
It is beyond doubt that the “public” referred to in the foregoing quote takes the form of the person skilled in the art.
Date modified: December 2010
The statutory requirements of proper disclosure are set out in subsection 27(3) of the Patent Act, which requires that:
The specification of an invention must
Thorson P. summarized the foregoing requirements in Minerals Separation North American Corp. v. Noranda Mines, Ltd.,[77] noting that
[t]he description must be correct; this means that it must be both clear and accurate. It must be free from avoidable obscurity or ambiguity and must be as simple and distinct as the difficulty of description permits. It must not contain erroneous or misleading statements calculated to deceive or mislead the persons to whom the specification is addressed and render it difficult for them without trial and experiment to comprehend in what manner the invention is to be performed. It must not, for example, direct the use of alternative methods of putting it into effect if only one is practicable, even if persons skilled in the art would be likely to choose the practicable method. The description of the invention must also be full; this means that its ambit must be defined, for nothing that has not been described may be validly claimed. The description must also give all information that is necessary for successful operation or use of the invention, without leaving such result to the chance of successful experiment, and if warnings are required in order to avert failure such warnings must be given. Moreover, the inventor must act uberrima fide and give all information known to him that will enable the invention to be carried out to its best effect as contemplated by him.[78]
The foregoing touches on both aspects of a sufficient disclosure: that it set out in clear and precise terms what the invention is (i.e. a correct and full description), and that it provide sufficient instructions to the person skilled in the art so that this person is enabled to reproduce and successfully operate the claimed invention.
Date modified: October 2019
The specification of an invention is directed to a person skilled in the art or science to which it pertains, or with which it is most closely connected.[79] Whether or not a description is sufficient depends on the interpretation it would be given by the person skilled in the art, who must interpret it with a mind willing to understand[80] and desirous of success.[81]
The person skilled in the art is competent, and represents an average, logical but unimaginative worker in the field.[82] This person is neither a dull-witted incompetent nor a creative, intuitive expert,[83] albeit that in a highly technical field the person skilled in the art may be presumed to have expert-level knowledge and skills.[84] Furthermore, the person skilled in the art is reasonably diligent in keeping up with advances in the field or fields of relevance to the invention,[85] and has the advantage of being multilingual and thereby being able to comprehend prior art in any language.[86]
In addition, the person skilled in the art need not be an actual individual; they are a fictitious construct and can represent a team of individuals whose conjoint knowledge is relevant to the invention in suit.[87]
In order to properly assess whether a correct and full description of the invention has been provided, it is necessary to identify the person skilled in the art to which the application is directed.
In accordance with paragraph 56(1)(b) and 56(1)(d) of the Patent Rules, the description must indicate the technical field of the invention and must describe the invention in a manner that permits the technical problem and its solution to be understood. The person skilled in the art will be competent in the field or fields of relevance to the invention.
A complexity arising from the nature of the person skilled in the art is that, as a general rule, neither the inventors nor the examiner may be directly equated to this person. Examiners and inventors, for example, are not free of creativity and intuition. They may have knowledge that surpasses that expected of the person skilled in the art in a given field, but again may not be as skilled in other fields of the invention as this person. During examination, an examiner must attempt to interpret the application and the prior art using the appropriate knowledge that the person skilled in the art would have possessed at the relevant date [see 14.02.03]. This may be particularly challenging where knowledge in the field at the date of examination has significantly developed since the relevant date, and particularly where certain views held at the relevant date have subsequently been found to be incorrect.[88]
Where the precise nature of the person skilled in the art is relevant for resolving an issue during examination, the examiner will determine who this person is and will take due account of any representations made by the applicant on point.
Date modified: December 2010
A description sufficient to allow the person skilled in the art to practice the invention with the same success as the inventor is said to be enabling. Since the person skilled in the art is the addressee of the description, it is not necessary for common knowledge to be comprehensively disclosed nor to teach to the person skilled in the art things that would be plainly obvious to them.[89]
The date at which the person skilled in the art brings their knowledge to bear on the application is the date on which the application came into their possession; that is to say, the publication date.[90]
Since the common general knowledge may develop between the filing date and the publication date, this theoretically means that a specification that was not enabling as filed could nevertheless, on the basis of more extensive common general knowledge, be enabling by the publication date. However, the invention must still be fully described as of the filing date, and the utility of the invention must have been established no later than at this date [see 19.01.02].
Date modified: December 2010
The person skilled in the art will read a description with a mind willing to understand and desirous of success. They will use their common general knowledge to supplement the description in order to successfully operate the invention, and will overlook obvious errors or omissions that can be readily corrected.[91]
Where, however, a description includes statements that direct the person skilled in the art to attempt to practice the invention in a manner contrary to their common general knowledge, the person skilled in the art will nevertheless follow these explicit instructions. Where the manner of operation so disclosed will in fact not work to achieve the promise of the invention, the description does not comply with subsection 27(3) of the Patent Act.[92]
[For guidance regarding misleading definitions in the description, see 14.05.03.]
Date modified: December 2010
The person skilled in the art can be called upon to perform routine experiments to ensure proper operation of an invention, but must be able to practice the full scope of the invention without undue burden or the need to exercise their inventive ingenuity.
If the person skilled in the art is called on to solve problems in such a manner that undue burden or an inventive step are required, the description is insufficient (and the attendant claims are unsupported).[93] The obligation of the patentee for proper disclosure in this sense was described in Rice v. Christiani & Nielsen as:
[h]e must so draft his specification, that a person having a competent knowledge of the industry concerned [...] will be able readily to ascertain from it the relation the invention bears to the existing knowledge in the industry, and so that one should not be called upon to do experimental work in order to discover how the invention may be made operative. There must be an open exposition by the patentee of everything that is necessary for the easy and certain procurement of the commodity for which the patent was granted. The patentee is not to tell a man to make an experiment but to tell him how to do the thing.[94]
H.G. Fox later described the relationship between the specification and the person skilled in the art as follows:
[t]he person to whom the specification is addressed is presumed to possess all the existing knowledge common to the art to which the invention relates; this knowledge he must bring to bear in interpreting the specification. But he is not required to exercise or to be possessed of more, and, if the specification contains something that necessitates the working out of a problem, the patent cannot be supported.
Where a specification describes an invention sufficiently clearly to enable a reasonably skilled workman to make use of it, even though some experiments are necessary, the patent will be good so long as those experiments do not require any exercise of the inventive faculty.[95]
In certain arts, it is common to describe an invention as relying on materials having certain required properties (a metal with a certain ductility; an insulator with a certain dielectric value, a molecule with a certain dipole moment), rather than by naming the materials explicitly. This is permissible as long as identifying those materials that have the required property does not require undue burden or inventive effort.
Requiring the absence of inventive effort implies that the solution to the problem being addressed must be readily apparent to the person skilled in the art (i.e. obvious). Solving a problem with a readily apparent solution is routine, and a description that requires the solving of such a problem could nevertheless be considered to be sufficient. The Courts have noted that it can be considered uninventive to engage in “routine testing to determine characteristics of known compounds, not undertaken for the purpose of ‘searching for something novel’, but rather for the purpose of verifying the actual attributes of already known compounds”
.[96]
While verifying the predicted or predictable properties of known compounds may therefore be considered to be routine,[97] “verification” means “confirmation” and determining the unexpected and unpredictable properties of new compounds is consequently not “verification”.[98]
This reasoning can be extended to disciplines other than the chemical arts by formulating the statement as: a certain amount of routine testing is permitted in order to identify suitable materials for operating an invention, presuming the person skilled in the art knows or has been taught the necessary properties, how to determine them, and broadly what existing materials are likely to possess them.
Example 1:
An invention describes a particular type of flange for connecting a plumbing fixture to a pipe, wherein it is necessary to construct the flange using a metal whose ductility is within a certain range. Identifying this operative ductility range is the discovery underlying the invention. Several metals having the necessary ductility are identified, and general teachings are given as to what types of metals are likely to have the necessary property. Testing ductility is within the common general knowledge of the person skilled in the art, and is routine.
Claim:
1. A flexible flange for connecting a plumbing fixture to a pipe, said flange comprising a metal having ductility in the range x-y and [...]
Analysis: The claim is given breadth by defining the flange in terms of a metal having ductility in the defined range, rather than in terms of specific operative metals. Whether or not the claim as defined is enabled depends on whether it can be operated without placing undue burden on the person skilled in the art. This depends on whether the person skilled in the art can readily identify suitable metals. Given that the person skilled in the art can test a given metal to determine whether or not it has the necessary ductility, that for many metals this data is already available in published references, and that the description suggests which metals are likely to be suitable, there is no invention in identifying metals that have the necessary property. Verifying the properties of known metals is “routine”, and the person skilled in the art has not improperly been presented with problems to solve.
Example 2:
An applicant asserts as their invention drug compositions having very uniform release profiles for the active ingredient. Certain embodiments are disclosed based on particular salts of protected cyclic amines, but the invention is claimed in terms of drug compositions having the beneficial release profile, and not in terms of drug compositions of the particular family of salts.
Claim:
1. A medicament having a release profile characterised by [description of the profile].
Analysis: Consider that the release profile achieved is an unexpected and very beneficial property of the specific salts disclosed. The description does not disclose what chemical properties of the salt led to the defined release profile, nor does it guide the person skilled in the art as to what other compounds may provide a similar result.
In order to operate the full scope of the claim, the person skilled in the art would have to solve the problem of identifying all the other salts that would lead to the same release profile. Since the identity of these other salts (presuming some may exist) is unobvious, an inventive step is associated with their identification. The description is insufficient to support the invention as broadly asserted.
Date modified: December 2010
As a general proposition, it is not necessary for the description to provide a theory as to why the invention operates as it does. The requirement is, simply, that the description teaches the person skilled in the art what the invention is and how to make it operate to provide the promised benefits.
Thus, as noted in Apotex v. Wellcome, “[i]t is generally not necessary for an inventor to provide a theory of why the invention works. Practical readers merely want to know that it does work and how to work it”
.[99]
This general proposition, however, has to be understood in an appropriate context. The Supreme Court thus added to the comment quoted above by stating, in respect of an invention relying on sound prediction, that “[i]n this sort of case, however, the sound prediction is to some extent the quid pro quo the applicant offers in exchange for the patent monopoly”
.[100] It can consequently be understood that if the utility of the invention is predicated on a sound prediction [see 19.01.03], and the line of reasoning depends on an understanding of the theory as to why the invention works, it may not be possible to properly express the line of reasoning unless this theory is disclosed.
Date modified: October 2019
As was noted by the Supreme Court in Apotex v. Wellcome, the granting of patents is “a method by which inventive solutions to practical problems are coaxed into the public domain”
.[101] Being a solution to a practical problem is what provides to the invention the practical utility necessary for patentability.
The description must put the person skilled in the art in a position to appreciate the nature of the problem being solved and the solution provided by the invention. Paragraph 56(1)(d) of the Patent Rules states that the description must include the following information:
a description of the invention must be set out in terms that permit the technical problem and its solution to be understood, even if that problem is not expressly stated
In order to solve a practical problem, the solution must be in a form that can interact directly with the physical world and, hence, that will itself enable a person skilled in the art to obtain the intended result or benefit. That is, a patent is given for “the means by which a result is obtained ... rather than the result itself”
.[102] These means must consist of one or several elements, where an element in this sense could be either a physical object (a machine, article of manufacture or composition of matter) or a step leading to a physical effect in an art or process.
The group of elements that are made use of to obtain the benefit of the invention may, in combination, be referred to as the “practical form” of the invention (i.e. the form in which the invention may be practised). The practical form includes all the elements required to provide the utility of the invention.
In order for the description to properly disclose the practical form, it must supplement the common general knowledge of the person skilled in the art so as to put the invention into the hands of this person. Any novel element must therefore be fully described, as it was necessarily not previously known. Also, those elements (new or old) the person skilled in the art would not have known to use in combination to achieve the objects of the invention must be described, not only individually but in the appropriate combination.
For the description to disclose a patentable invention, it must describe (and the claims define) all the elements necessary to provide the useful result in a novel and inventive manner, and without which elements the solution would cease to be inventive.[103]
It is also necessary that the description provide such instructions as are necessary for the person skilled in the art to understand, where applicable, the interrelationship of the elements necessary to provide the practical form of the invention. The invention must be described so that, colloquially speaking, “the wheels will go round”, [104] and must not require that the person skilled in the art perform modifications to the invention described in order to make it work.[105]
Although external documents may be referred to in the description, the invention must be described and enabled by the description alone as interpreted by the person skilled in the art in view of their common general knowledge. Specific prior art knowledge (e.g. information only available in one or a few documents, and which has not been shown to be commonly known and accepted) may be considered not to be “common general knowledge”, and in such cases those specific teachings from the prior art necessary to describe or enable the invention must be included in the description in order to provide a full and complete disclosure.
It is not necessary to supplement a description of the foregoing with a description of those elements that would be self-evidently necessary to operate the invention, and whose use in the context of the invention as described would be obvious to the person skilled in the art.[106]
During prosecution, amendment to the claims may appear to alter the nature of the invention. Care must be taken to ensure that the inventor was, no later than the filing date, in possession of the invention asserted in the amended claims. Inventive ingenuity cannot post-date filing.[107] This is particularly relevant where features not identified in the original specification as being related to specific advantages are subsequently asserted as rendering the claims non-obvious over prior art disclosures.
It is important to consider whether the description teaches that the elements in question are simply optional, or are essential elements of preferred embodiments. Where the inclusion of an element will lead to additional benefits over the invention as broadly disclosed, it should be viewed as an essential element of the “narrower invention” (the subject-matter in a claim of narrower scope).
Date modified: December 2010
A combination, in the sense the term is used herein, is an assemblage of parts (often of known parts) whose conjoint use leads to a result that is “different from the sum of the results of the elements” that make it up and “that is not attributable to any of the elements but flows from the combination itself and would not be possible without it”
.[108] Such a result may conveniently be termed a “unitary” result.[109]
A patentable combination has been explained in the following way:
it is accepted as sound law that a mere placing side by side of old integers so that each performs its own proper function independently of any of the others is not a patentable combination, but that where the old integers when placed together have some working inter-relation producing a new or improved result then there is patentable subject matter in the idea of the working inter-relation brought about by the collocation of the integers.[110]
Where several parts are used together, each providing its expected result and the whole not leading to a unitary result, the assemblage is referred to as a “mere aggregation”,[111] or simply as an “aggregation”, to distinguish it from a true combination.
The utility of a combination is the unitary result it provides, and it is this result that must be established by demonstration or sound prediction.
Where, having described the structure of the combination, it would not be clear to the person skilled in the art what unitary result it achieves, a correct and full description of the result itself may be necessary to show that the combination is useful and inventive and to distinguish it from a mere aggregation.
Date modified: December 2010
The following sections set out practice in respect of certain specific topics which give rise to particular considerations with respect to proper disclosure.
Date modified: December 2010
In certain cases, applicants may wish to describe or define an invention using functional language. The use of functional language, whether in a claim or in the description, is not per se objectionable. Such language, however, is generally used to provide breadth and must be carefully considered from the perspective of proper support.
Functional limitations must always be considered from the perspective of the person skilled in the art, with the question to be asked being: “can the person skilled in the art practice, in view of the description, the full breadth of the claimed invention without recourse to undue experimentation or inventive ingenuity?” [see 14.02.05]. If the means to effect the defined function are common general knowledge, the functional limitation is unlikely to be objectionable. Where few or only one means is known to effect the function, however, broad functional language would direct the claimed invention to be practised in ways that have not been fully described or enabled and consequently would be objectionable.
Typically, the inquiry into the appropriateness of functional language is driven by the language of the claims. Where an invention is defined in terms of an overly broad functional limitation, the claim seeks to monopolize speculative embodiments that the inventors have not adequately described. The corollary is that the description is not sufficient to support the invention as claimed.
To paraphrase Free World Trust v. Électro Santé Inc., it is not legitimate to invent a particular composition that grows hair on bald men and thereafter claim all compositions that grow hair on bald men.[112]
Thus, a claim to “a composition comprising a hair-growth activating compound in a pharmaceutically acceptable carrier”
, where only compound X is known to provide the function, would be too broad. The limitation “hair-growth activating” is a functional limitation to the scope of the compounds found in the composition, but does not serve to make the scope of the claim clear to the person skilled in the art. Identifying all the compounds that would have this activity would require extensive inventive experimentation amounting to invention [see 14.02.05]. The description, therefore, is not sufficient to describe and enable the invention asserted in the claim, and is objectionable under subsection 27(3) of the Patent Act.
In contrast, if it had been discovered that the combination of a particular drug with any non-steroidal anti-inflammatory (NSAID) compound led to unexpected advantages, functionally limiting the scope of the second component of the composition by the limitation “NSAID” would not be problematic. The scope of the term “NSAID” (or “NSAID compound”) would be immediately apparent to the person skilled in the art.
Similarly, in a mechanical invention that relies on a “cutting means”, a number of different cutting means would be known to the person skilled in the art. Where it would be readily apparent which would be suitable for operating the claimed invention, the limitation “cutting means” would not improperly broaden the claim. The identification and selection of appropriate cutting means would not require undue effort or further invention in such a circumstance.
Date modified: December 2010
Specific disclosure requirements exist for some inventions in the fields of biotechnology. In brief, it may be necessary for a sequence listing of a nucleotide or amino acid sequence to be included with the description or for a deposit of biological material to be made with an International Depository Authority in order for the description of a biotechnology invention to be considered to be sufficient.
Details on the requirements for providing sequence listings or deposits of biological material are provided in subsection 23.05.07 and 23.06, respectively, of this manual.
Date modified: December 2010
It has long been understood that the language of the claims is to be construed in view of the specification as a whole, and that the applicant can serve as their own lexicographer.
Their Lordships do not doubt that it is possible for a patentee to make his own dictionary in this way. If he has put something in the earlier part of the specification which plainly tells the reader that for the purpose of the specification he is using a particular word with a meaning which he sets out, then the reader knows that when he comes to the claims he must read that word as having that meaning. But this is an awkward method of drafting and is very undesirable where a simpler method could easily be adopted and it is in all cases incumbent on a patentee who chooses to adopt this method to make his intention plain to those who read the specification.[113]
During examination, the language of the claims is interpreted by giving each term its plain and usual meaning in the art to which the invention pertains, unless it is clear from the description that a term in the claims is to be given a different meaning.
In the context of proper disclosure, it is to be noted that where an applicant, in attempting to act as their own lexicographer, creates a definition for a term that is contrary to the usual meaning ascribed to that term in the art, that is liable to cause confusion or ambiguity, or that is unnecessary in that other plain language could as easily provide the same information, the definition is objectionable. Recall in this context the requirement discussed in 14.02.01 that “[t]he description must be correct; this means that it must be both clear and accurate. It must be free from avoidable obscurity or ambiguity and must be as simple and distinct as the difficulty of description permits”
.
For example, where the description teaches that, for the purposes of the invention, the symbol P (phosphorus) designates nitrogen (elemental symbol N), this definition is only liable to cause confusion and is objectionable under subsection 27(3) of the Patent Act. The symbol is recognized in chemistry as designating phosphorus, and could readily be replaced by the appropriate symbol, N, to designate nitrogen.
In contrast, a definition is generally acceptable if, for the purposes of expediency and without sacrificing clarity, it narrows the scope of a term that would otherwise be attributed a broader meaning in the art. In a given case, it might be acceptable to define, for example, that the term “ethylene polymer” means “a non-crosslinked polymer comprising at least 80 mol% ethylene, with up to 20% C3-8 alkene comonomer”
. Providing the longer definition at multiple instances would be unnecessarily cumbersome, and the definition provided unambiguously restricts the broader term.
Date modified: December 2010
An invention may be operated by way of trademarked products. Simply naming a trademarked product is not, however, equivalent to describing the composition of that product.
Further, simply knowing what components are included in a trademarked product does not identify which of those components is an essential element of the invention (i.e. which component or components are necessary to fulfill the trademarked product’s role in the invention). Thus, even though a person skilled in the art may, depending on the state of the art, be able to reverse engineer a trademarked product and identify its components, this will not by necessity put them in possession of the invention.
Therefore, where an invention is described only in terms of a trademarked product, the question of proper support must be carefully considered. If it is not clear which component of the product is responsible for the product’s role in the invention, the invention cannot be operated other than by the trademarked product itself.
If the composition of the trademarked product is not known, and the product is not commercially available, the invention is not enabled.
Where an invention is described in terms of specific components (e.g. chemical compounds), but is supported by examples that rely on trademarked products of undisclosed composition, no presumption exists that the examples embody the invention described. The applicant must establish that they were aware of the composition of the trademarked product no later than at the filing date.
Where the composition of a trademarked product did not form part of the prior art as of the filing date, its composition cannot subsequently be added to the application [see 14.08].
[For requirements regarding the identification of trademarks, see 14.07.03.]
Date modified: October 2019
The invention must be “correctly and fully” described in the description, which according to subsection 1(1) of the Patent Rules is “the part of the specification other than the claims”. Furthermore, in accordance with section 60 of the Patent Rules, the claims must be fully supported by the description.
It is consequently improper for the description to state the nature of the invention by reference to the claims. Such statements suggest that the description does not “correctly and fully” disclose the invention and does not comply with subsection 27(3) of the Patent Act.
Therefore, where the description teaches in some fashion that the invention is “according to the claims”, the statement must be removed or replaced by an explicit description of the invention.
By way of example, statements such as “the problem of premature ignition in the combustion chamber is overcome through the method of claim 1”
or “the compositions as instantly claimed exhibit superior insecticidal properties”
fail to set forth explicitly what the invention in question is, but suggest instead that the invention is whatever might be claimed at any given moment in time.
Note that amending the description of a non-divisional application, to include the language of the claims originally filed is necessarily compliant with subsection 38.2(2) of the Patent Act.
This subsection has been deleted.
Date modified: December 2010
Where an application includes a statement whose correctness is dependent on foreign patent prosecution practices or laws, such a statement may be inaccurate or liable to cause confusion in the context of Canadian law. Where this is the case, the statement must be removed. The statements may be viewed as being “incorrect”, and therefore a defect under subsection 27(3) of the Patent Act [see 14.09].
An indication that the application is a continuation-in-part or a divisional of a foreign patent document, for example, is not correct in the context of the Canadian Patent Act and must be removed.
A statement regarding the rights of foreign governments to the invention may also be misleading, and should be removed if it is inaccurate.
Date modified: October 2019
The form a description should take is set out in section 56 of the Patent Rules. Thus,
(1) The description must include the following information, set out in the following manner and order:
(a) the title of the invention must be stated in a short and precise manner and must not include a trademark, coined word or personal name;
(b) the technical field to which the invention relates must be specified;
(c) the background art that, as far as is known to the applicant, is important for the understanding, searching and examination of the invention must be described;
(d) a description of the invention must be set out in terms that permit the technical problem and its solution to be understood, even if that problem is not expressly stated;
(e) the figures in the drawings, if any, must be concisely described;
(f) at least one mode contemplated by the inventor for carrying out the invention must be set out using examples, if appropriate, and with reference to the drawings, if any; and
(g) a sequence listing, if required by subsection 58(1), must be included.
(2) The description may be presented in a different manner or order if, because of the nature of the invention, a different manner or order would result in a better understanding or more economical presentation of the invention.
The provisions of subsection 56(2) of the Patent Rules would allow, for example, that drawings associated with the prior art be described with the background art, prior to the brief description of the figures in any remaining drawings.
The title of the invention should be descriptive of the invention in suit, and not merely of the field to which the invention pertains. A title such as “flame-retardant rigid polyurethane foam” is acceptable, whereas “foam” is not.
In accordance with paragraph 56(1)(a) of the Patent Rules, the Office considers the title provided in the description to be the correct title of the invention. Where, for any reason, the title ascribed to the invention in the Office’s electronic database differs from the title provided in the description, the electronic database will be updated at the time of grant to reflect the title set out in the description.[114]
Disagreement between the title in the description and the title in the Office’s electronic database is not a defect in the application. An examiner may note the existence of such a disagreement, in order to apprise the applicant of the situation and provide them with an opportunity to address the matter. Such a disagreement may also be brought to the applicant’s attention subsequent to allowance, by way of an Office letter.
Paragraph 56(1)(c) of the Patent Rules requires that the applicant describe the background art that, as far as is known to them, is important for the understanding, searching and examination of the invention. Where relevant background art is identified during prosecution, an applicant may, within the limitations imposed by section 38.2 of the Patent Act [see 14.08], introduce to the description references to and descriptions of the contents of prior art documents where these are clearly admitted to be prior art with respect to the application. Examiners should, in general, not raise an objection simply because the description has not been amended to identify background art brought to the applicant’s attention subsequent to filing.
Paragraph 56(1)(f) of the Patent Rules provides that, “if appropriate”, the applicant must set forth in terms of examples, at least one mode contemplated by the inventor for carrying out the invention. The use of the wording “if appropriate” in this rule reflects that an exemplary basis may or may not be necessary depending on the case at hand. The language “if appropriate” does not merely mean “if the applicant deems it appropriate”, and does not provide any exception to the disclosure requirements of subsection 27(3) of the Patent Act.
It is not necessary for the description to present the information required by section 56 of the Patent Rules in sections bearing headings corresponding to the paragraphs of subsection 56(1), although an applicant may choose to do so for the sake of clarity.
Headings such as “Summary of the Invention”, “Detailed Description of the Invention” and “Detailed Description of the Preferred Embodiments” are permitted in Canadian practice. It is worth noting, however, that where a heading such as “Detailed Description of the Preferred Embodiments” is used, support for claims broader than these embodiments must be found in other parts of the description which must satisfy the requirements of subsection 27(3) of the Patent Act, including enablement and support for any sound prediction, in respect of the invention as broadly claimed.
Date modified: December 2010
The description is subject to many formalities requirements dealing with various aspects of its contents and presentation. These are summarized in the following sections.
Date modified: October 2019
In accordance with subsection 50(1) of the Patent Rules the pages of the description, as part of the specification, must be numbered consecutively, and in accordance with section 49 of the Patent Rules no page of the description may contain the petition, the abstract, any drawings nor the claim(s), as each of those parts of the application must begin on a new page.
Date modified: October 2022
In accordance with section 51 of the Patent Rules, the description must not contain drawings[115] but may contain chemical or mathematical formulae.[116] For greater clarity, a chemical formula may be presented in the description in graphical form (i.e. as a structure).[117] The description may also contain information presented in tables. In accordance with subsection 14(2) of the Patent Rules, any formula or table may, where it aids presentation, be presented sideways (i.e. in landscape format). Otherwise, subsection 14(1) of the Patent Rules provides that pages of the description must be used upright (i.e. in portrait format).
It can be inferred from subsection 27(5.1) of the Patent Act that a drawing is an illustration of the invention. Schematics that illustrate a process, such as flow-charts, are generally considered to be drawings.
Graphical representations of data, such as graphs, histograms, pie charts, and spectra, are not necessarily to be viewed as “illustrations of the invention”, and therefore may be included in the description. Where a graphical representation of data is provided as a drawing, it must comply with all the requirements of section 59 of the Patent Rules.
Tabulated data generally cannot be considered a “drawing”, and typically should be presented in the description.
Where the application contains drawings, subsection 59(11) of the Patent Rules requires that any reference characters appearing on any figures in the drawings (including photographs), and only these reference characters, be mentioned in the description. Further, subsection 59(12) of the Patent Rules requires that when a reference character is used for a particular feature, that it be the same throughout the abstract, specification and drawings. The same reference character cannot be used to refer to any other features.
Date modified: October 2019
In accordance with section 52 of the Patent Rules, any trademark mentioned in the abstract, specification or drawing must be identified as such. The Office requires that each trademark be identified in an appropriate manner at least once, preferably at its first appearance.
Use of the words “trademark” in parentheses, of the designation “™”, or of an indicator such as an asterisk (*) linked to a footnote denoting that the asterisk designates a trademark are all examples of appropriate manners for identifying a trademark.
Date modified: October 2019
In accordance with section 57 of the Patent Rules, a document referred to in the description must be available to the public and be fully identified, and must not be incorporated by reference.
The Office considers a patent document to be properly identified when the country or office code is provided along with a number under which the published version of the document can be found. Thus, the number provided can be that given to a granted patent, or be either the application number or publication number of a published application.
WO 96/937212, US 5,410,288, and EP 1 004 793 are examples of patent documents properly identified by a publication or patent number.
PCT CA2006/001,285 and U.S.S.N. 11/421,399 are examples of application numbers which are acceptable if the identified application has been published.
PCT applications, and US applications filed after November 28, 2000, will generally be published unless the application has been withdrawn (or, in the case of US applications, abandoned) prior to the publication date. Under 35 U.S.C. 122, a US application may also be kept confidential (i.e. will not be published) if the applicant certifies that they will not file an application for the disclosed invention in any other country. Where a US application is relied on as a priority document, this provision does not apply. US provisional applications, applications for design patents, and applications in series 09 or earlier are not necessarily published and may not be referred to by their application numbers unless the document is available to the public.[118]
For non-patent documents, the requirement is that the document be sufficiently well identified to permit it to be obtained by an interested party.
For a journal article, textbook, or the like, the document should be identified by the names of the author and the publication, the year of publication, the volume and/or issue number(s) if applicable, and the page numbers of the article, number of the chapter or the like. Preferably, the title of an article or title of a chapter should be provided. Additional information, such as the name of the publisher, may be included. Where a unique document identifier such as an ISBN code is provided, this does not replace any of the foregoing requirements.
References to internet pages present a particular difficulty in that neither the URL nor the content of such pages is necessarily fixed. Examiners will object to the identification of a document by way of a URL where it is not clear that the URL refers to a reliable, publically available source that can reasonably be expected to ensure the information in question is of fixed content and will be more or less permanently retrievable.
Date modified: October 2022
General guidance regarding the amendment of applications is provided in chapters 11 and 20 of the manual.
Note that one amendment that is always permissible from the standpoint of “new matter” is the inclusion of the language of the originally filed claims in the description of a non-divisional application that was originally filed in English or French.
As regards the description, particular attention must be given to amendments that replace restrictive language with permissive language. Where an application teaches that the invention (as opposed to an embodiment of the invention) “must be” or “is” (or the like) operated in a certain way, amendment of this language to indicate that the invention “preferably” or “optionally” (or the like) is operated in that way enlarges the scope of the invention and may be seen as the addition of new matter.
Similarly, it is possible for the deletion of text to amount to the addition of new matter. If a passage in the description teaches that an invention is inoperative under certain conditions, an amendment to remove this guidance could be viewed as introducing new matter by expanding the scope of the operable invention.
Where a description included both permissive and restrictive language regarding a certain limitation, amending the description to make it self-consistent throughout will generally not be seen as the addition of new matter.
An invention requires an inventive step, and the presence of this inventive step must be evaluated in view of the specification as filed. Amendments that appear to introduce new aspects of “inventiveness” to the application introduce new matter.
Remembering that an invention is a solution to a practical problem, it can be understood that amendments that tend to transform the invention as originally disclosed into a new invention – that is to say, into a new solution to the same or a different problem – constitute the addition of new matter.
Such amendments shift the point of invention and have the effect of causing a different invention to be disclosed than that in the specification as originally filed.
The description of an application may be amended to make reference to prior art documents. Where the amendment is merely to clarify the state of the art, this will generally not be considered to introduce new matter. Where, however, an amendment introduces information from a prior art document, these amendments may, depending on the circumstances, introduce new matter.
Where specific teachings in a prior art document are required in order to enable the invention to be operated, or in order to support a sound prediction of utility, and it would not have been clear to the person skilled in the art, as of the claim date, which teachings in the prior art document were necessary for this purpose, identifying or including the specific teachings constitutes the addition of new matter.
Date modified: October 2019
Objections dealing with substantive issues of sufficiency are presented under subsection 27(3) of the Patent Act, or a specific paragraph of that subsection where this precision may be helpful in underlining the basis of the objection.
As is the case with objections under subsection 27(4) of the Patent Act, however, the defects being objected to under subsection 27(3) can range from significant issues of sufficiency to fairly minor defects of clarity. The presence of a subsection 27(3) objection is not by necessity an indication of any un-remediable defect relating to sufficiency.
Nevertheless, wherever a more specific authority exists on which to base the objection being made, this authority should be used in place of subsection 27(3) of the Patent Act. For example, if a reference character has been included in the drawings but is not mentioned in the description, this defect should be presented under subsection 59(11) of the Patent Rules rather than under subsection 27(3) of the Patent Act.
Objections to formatting or other minor problems are presented under authority of whichever section relates to the defect under consideration [see 14.07 and the related endnotes].
Non-compliance with the formatting requirements set out in sections 13, 47 and 48 of the Patent Rules [see sections 4.09 and 11.04.04 and chapter 34 of this manual] can be identified by an examiner in order to inform applicants of any defects and expedite advancing the application to allowance. It is not, however, required for an examiner to do so, since correction of these defects can also be requisitioned non-compliance can also be identified by examination support staff. It is noted that the Canadian requirements as to formatting are based on those required under the Patent Cooperation Treaty, and requisitioning compliance with the Canadian requirements is therefore permissible under Article 27, PCT.
Date modified: October 2022
Inventions which can be illustrated by means of drawings shall be so illustrated in an application for a patent. The role of the drawings is to clarify the principles of the construction of a device rather than to provide particular details of dimensions or relative proportions. The drawings must clearly show all parts of the invention (subsection 27(5.1) of the Patent Act) and must be without colouring (subsection 59(3) of the Patent Rules). Known devices may be illustrated by symbols which have a universally recognized conventional meaning provided that no further detail is essential for understanding the subject-matter of the invention. Where text matter in the drawings would give a better understanding of the drawings, a single word or a few words may be used. Blank “blocks” in schematic diagrams must be descriptively labelled. Figures in the drawings which illustrate the prior art should be labelled “PRIOR ART”.
Each drawing must include reference characters corresponding with those in the specification, and the Commissioner may require further drawings or dispense with any of them as the Commissioner sees fit (subsection 27(5.2) of the Patent Act). If a notice for further drawings under subsection 27(5.2) of the Patent Act is not replied to, in good faith, within 3 months, the application will be deemed abandoned under paragraph 132(1)(c) of the Patent Rules.
Whenever drawings are provided in an application, they must conform to the provisions of sections 46, 49 and 59 and subsections 14(2)and 47(2) of the Patent Rules. Subsection 56(2) of the Patent Rules permits reference to the drawings before they are concisely described when the reference is made in respect of prior art.
Date modified: October 2022
Subsection 38.2(1) of the Patent Act states that the specification and any drawings furnished as part of an application may be amended before the patent is issued.
Drawings may not be amended to add new matter.
Detailed information on making amendments to patent applications can be found in Chapters 11 and 20 of this manual.
Date modified: October 2019
In any case in which an invention does not admit of illustration by means of drawings but does admit of illustration by means of photographs, the applicant may, as part of the application, furnish photographs that illustrate the invention (subsection 59(2) of the Patent Rules). Any such photograph can contain colour (subsection 59(3) of the Patent Rules) but is still subject to subsections 59(3)-(13) of the Patent Rules. Further, while Canada is a Receiving Office (RO) that does allow for colour in photographs, not all other international receiving offices do. Some ROs may convert colour photographs into black and white. Care should be taken since any converted photographs may lose detail in the photograph and any addition of such detail after the filing date can be considered new matter.
Date modified: March 1998
In order to fulfill their public notice function, a claim must define the invention in such a manner that the person skilled in the art will understand where they may and may not go without infringing.
As Lord Loreburn noted in Natural Colour Kinematograph Co v Bioschemes Ltd, “[t]he patent system is designed to advance research and development and to encourage broader economic activity. Achievement of these objectives is undermined however if competitors fear to tread in the vicinity of the patent because its scope lacks a reasonable measure of precision and certainty. A patent of uncertain scope becomes a public nuisance”
.[119]
The claims, therefore, must define distinctly and in explicit terms the subject matter of the invention for which protection is sought (section 27(4) of the Patent Act). Patentable claims must define novel subject matter. To be considered novel the whole of subject matter defined by a claim shall not form part of the state of the art. With respect to each claim in an application for patent in Canada the state of the art may be defined generally as everything disclosed in such a manner that it became available to the public in Canada or elsewhere before the CLAIM DATE. The CLAIM DATE of a claim in a Canadian patent application is the filing date of the application in Canada, unless, priority is claimed on an earlier filed application in Canada or elsewhere. In the latter case, the claim date is the filing date of the earliest application which supports the subject matter of the claim. See sections 2 and 28.1 of the Patent Act and section 18.03 for more detail. The claims should also specify in a positive manner all the elements, features, and critical aspects of the invention which are necessary to ensure the result as set forth in the description. Each claim (read with the introduction to the claims) must be restricted to a single sentence.
Claims may be drafted to contain the following three parts:
The preamble identifies the category of the invention and may state the purpose of the invention with regard to this category.
Examples:
A machine for waxing paper ...
A composition for fertilizing the soil ...
The transitional phrase joins the preamble to a recitation of the elements of the invention to be protected. It also indicates, in an abbreviated way, whether the recitation is left open or closed to additional elements.
Examples:
which comprises, comprising, including, having ...
consisting of, consisting essentially of ...
The body of the claim lists the main elements of the invention, such as, parts of a device, steps of a process or method, ingredients of a composition, or groups in the chemical formula of a compound.
Notwithstanding the above, the Patent Office will accept any form of claim that conforms to section 27(4) of the Patent Act and that sets forth an invention in distinct and explicit terms and otherwise conforms to the Patent Act and the Patent Rules.
For a consideration of claims with respect to the prior art (novelty and non-obviousness) see Chapter 18.
For consideration of claims with respect to utility, operability and non-patentable subject matter (section 2 of the Patent Act) see Chapters 17 and 19.
Date modified: March 1998
Claims are the starting point for construing a patent as they define the invention and exclusive right sought. The relevant date for the analysis of a claim is the claim date (see section 18.03). When construing a claim the essential elements must be determined. However, in order to determine the nature of the invention and the essential elements of the invention, the specification must be construed as a whole. Analysis of a patent is to be determined from the point of view of one skilled in the art, with a mind willing to understand the invention.
Even though claims are construed with reference to the description, reference to the description is only permitted to assist the understanding of terms used within the claims if these terms have a unique meaning. Reference to the description is not permitted for terms that have a plain, common, and unambiguous meaning as these terms would be known to someone of skill within the art, nor is reference to stray phrases within the description considered support for terms within the claims. Furthermore, reference to the description cannot be used to vary the scope of the claims.
During examination, the language of the claims is interpreted by giving each term its plain and usual meaning in the art to which the invention pertains unless it is clear from the description that a term in the claims is to be given a different meaning.
As mentioned above, the courts have acknowledged that an applicant can act as their own lexicographer, by specifying in their description that certain terms will have particular meanings for the purposes of the application. Whenever an applicant desires to act as their own lexicographer; however, it is incumbent on them to make this clear from the language of the description. Further, in so acting it is not proper to give a term having a well-known meaning a definition which is contrary to this meaning. In such cases, uncertainty exists as to whether the term, when found in a claim, is intended to have its usual or distorted meaning.
For example, teaching that the term “up” means “down” for the purposes of the invention is only liable to cause confusion and serves no purpose. Such a definition, when made in the description, would be objected to under subsection 27(3) of the Patent Act. Further, the claim containing the term “up” is objected to under subsection 27(4) of the Patent Act for the lack of clarity as to whether the term is intended to actually mean “up”, or rather to mean “down” following the teachings of the description. Similarly, teaching that the symbol “P” indicates nitrogen atoms is misleading; the symbol is recognized in chemistry as designating phosphorus, and could readily be replaced by the appropriate symbol “N” to designate nitrogen. In contrast, teaching that the term “protein”, for the purposes of the invention, has some specific but sensible meaning could be acceptable, especially where this avoids having to repeatedly include a lengthy definition in the claims.
Whenever inclusion of the definition found in the description into the claims would not be detrimental to the clarity and conciseness of the claim; however, this should be done.
It is worth noting that the courts, in construing the claims of a patent, are dealing with a document whose language is fixed. Any deficiencies in the language of the claim can only be remedied by construing the claim in “an informed and purposive way”. During examination, in contrast, the language of the claims may be amended so as to remove ambiguity and maximize their usefulness in serving their public notice function of defining the extent of the monopoly sought.[120]
Where a defect of clarity has been noted by an examiner in the language of a claim, it will generally be maintained in the face of a response arguing that the courts could, with the assistance of expert testimony, arrive at some construction thereof. The purpose of the claims is to serve a public notice function, and “nothing can excuse the use of ambiguous language when simple language can easily be employed”
.[121]
The application of these principles can be found in the following: Beecham v Procter Gamble 1982; AT &T v Mitel 1989; Airseal v M&I Heat 1993; Hi-Quail v Rea's Welding 1994; Mobil Oil v Hercules 1994; Cochlear v Cosem; and Almecon v Nutron 1996.
Date modified: March 1998
No speculation should be necessary to determine what is covered by each claim. It must not define some parts of the desired monopoly while only alluding to or vaguely mentioning others. If the invention is difficult to claim, due allowance is given for the limitations of language but involved language should not be used when the invention can be claimed simply. Wording should not be so flexible that several interpretations of it are possible, i.e. the claim should not have more than one meaning or be capable of both broad and narrow interpretations.
An identification of a claim having a defect for ambiguity or lack of clarity as to its limits (indefiniteness) is made under subsection 27(4) of the Patent Act. A claim is not indefinite simply because it is broad, but rather where the precise limits of the claim are uncertain. A claim that relies, for example, on the use of “a polyol” is not indefinite since the person skilled in the art can immediately appreciate the scope of that term. A claim relying on “a polyol capable of <performing some function>”, however, is indefinite if the person skilled in the art would not know, or be able to reasonably predict or determine, what polyols fall within the scope of the claim.
Date modified: March 1998
When an element is referred to in definite terms without having been introduced previously, the claim is objectionable under section 27(4) of the Patent Act. An example of this is, "A device for cracking nuts comprising a cup shaped base and a striker element, said lever tripping the hammer at timed intervals"
. In this claim there are no proper antecedents for "said lever" and "the hammer".
Implied antecedents may be permitted where the word or phrase, by definition, always contains the missing antecedent. For example, a claim beginning with: "A wheel, the axis being..." or "A compound having the formula I..." are acceptable.
Date modified: March 1998
The claims must be framed in distinct and clear language. They should not include vague or equivocal forms of wording which will create doubt. Examples of unclear language are relative terms or expressions such as "thin", "strong", "a major part", "if desired". If such expressions appear in a claim, they must be further defined in clear and distinct terms or be removed from the claim.
The following are some of the most commonly used imprecise terms that may be encountered in claims:
Other terms which in certain circumstances may be indefinite are:
Whenever any of the above terms is encountered in a claim, a possibility exists that the claim may not satisfy the requirements of the Patent Act and Rules. Specifically, subsection 27(4) of the Patent Act and Section 60 of the Patent Rules should be considered.
Some of these terms have been considered in decisions by the courts or by Commissioner's decisions.
a) "Containing as an active ingredient"
This phrase should, in some circumstances be refused as being ambiguous and indefinite because "an" implies the presence of other unspecified active ingredients in addition to the one specified in the claim.
Note: This phrase would be acceptable in a claim if "an" is changed to "the" and the other ingredients of the composition are specified while the utility for which the composition is intended is either inherent from the wording of the claim or expressly stated therein (Rohm & Haas v. Commissioner of Patents 30 C.P.R. 113, Ex.C.).
b) "Therapeutically effective amount"
As was stated in Gilbert v. Sandoz 64 C.P.R. 14, Ex.C., this is an ambiguous term in a claim. The claims in suit included this phrase in conjunction with a particular phenothiazine derivative when produced by specified process claims in association with a pharmaceutical carrier. While it is recognized that the essence of a great many inventions based on compounds for medicinal purposes resides more in the discovery of the unexpected medicinal utility of the compound than in its effective dose, nevertheless, when such a functional statement occurs in a claim, the medicinal utility of the composition of matter must be stated or be inherent from the preamble of the claim.
A particular amount of an active ingredient in combination with another compound (X) may have an entirely different therapeutic value than a very different amount of the same active ingredient in combination with compound X. Therefore, this functional phrase should only be permitted in a composition of matter claim when the utility of the composition of matter is indicated in the claim and provided that the actual amount taught and prescribed in the disclosure is not an important aspect of the invention. This amount may vary over a considerable range apparent to one skilled in the art because of similar known ranges for analogous compounds for the same purpose. However, if the disclosed range is an important feature of the invention or if the invention is only operable within a prescribed range so as to produce the promised results, then of course this disclosed range must be included in all of the independent claims.
This is an acceptable phrase in a claim if it is used in relation to one part of a two-part system where it is clear that it means more than 50%. However, when it refers to one part in a system consisting of three or more parts, it is refused as indefinite because it is not clear if it means a greater percentage than any of the other components or more than 50% of the overall total.
Date modified: March 1998
Claims containing negative expressions such as "not being...", "not having...", "not requiring..." may be objectionable under section 27(4) of the Patent Act in that claims should generally set forth what the invention is or does, and not what it is not or does not do, unless there is no positive way to describe it. Sometimes a dependent claim (Chapter 16.06) contains provisions which effectively cancel or negate some of the features of a preceding claim, thus making the dependent claim broader than the preceding claim. This is objectionable under subsection 63(4) of the Patent Rules.
Date modified: March 1998
To define the invention distinctly and in explicit terms, it is required that sufficient elements be recited for operability. The inventive features must appear in each claim. In the case of a composition, a claim must define a minimum of two ingredients, at least broadly. If a claim does not do this, it is objected to as indefinite and contrary to subsection 27(4) of the Patent Act.
Date modified: October 2019
A claim must be fully supported by the description as required by section 60 of the Patent Rules. All the characteristics of the embodiment of the invention which are set forth in the claim must be fully set forth in the description (Section 60 of the Patent Rules). However, since any claims included in the application at the time of filing are part of the specification (see subsection 27(4) of the Patent Act and the definition of “description” in subsection 1(1) of the Patent Rules), any matter in the originally filed claims that was not included in the description as filed may be added to the description (except for divisional applications which have further requirements regarding new subject-matter see section 20.04 for more details).
A claim is objected to for lack of support by the description if the terms used in the claim are not used in the description and cannot be clearly inferred from the description. Terms used in the claims and in the description must be used in the same sense.
Date modified: March 1998
It is generally not acceptable for a claim to contain reference to the description or drawings (subsection 62(1) of the Patent Rules). However, in some instances, if the claim itself is complete and can be read and understood without the reference, the claim is acceptable. The claims must not, in respect of the technical features of the invention, rely on references to the description or drawings except where absolutely necessary. In particular, they must not rely on references such as: "as described in the description" or "as illustrated in Figure 3". The following are examples of exceptions:
Reference numerals used in the drawings are permissible in a claim provided they are between parentheses (subsection 62(2) of the Patent Rules), and the claim is otherwise explicit and complete. However, if a claim is not complete without referring to the parts of the drawings identified by numerals in parentheses, it must be objected to as contravening subsection 27(4) of the Patent Act.
Tabulations in the form of charts often appear in the descriptions of applications. Such tabulations may also be included in the drawings as are graphs, phase diagrams, absorption spectrograms and the like. In circumstances where the nature of the invention is very complex and it is practically impossible or extremely cumbersome to define the scientific relationship of the different factors in a precise and distinguishing manner, without making reference to other parts of the application, then reference to charts, graphs or tables may be permitted in the claims. However, if such a chart or table, for example, is brief and concise, i.e. about 5-10 lines, the applicant may be required to enter it into the claims (subsection 62(1) of the Patent Rules).
If a test can be accurately defined in a few lines, then it must be included in the claim and a mere reference to such a test as described should not be permitted. However, when such a test is complex and lengthy to describe, for example if it requires more than one page of the description to characterize it, then the applicant may make reference to the test as therein defined rather than reproduce the test in the claim.
Reference may be made, within a claim, to sequence listing identifier numbers and biological deposit catalogue numbers (subsections 62(3) and (4) of the Patent Rules). These procedures are specified in detail in chapter 23 (Biotechnology).
Date modified: March 1998
A claim may be as narrow as the applicant wishes within the scope of the invention disclosed. It must not, however, be broader than the invention as described or supported by the description. Furthermore, a claim will fail if, in addition to claiming what is new and useful, it also claims something that is old or useless (Minerals Separation v. Noranda Mines 12 C.P.R. 99; 12 C.P.R. 182; 15 C.P.R. 133).
Each claim must be read giving its words the meaning and scope which they normally have in the relevant art, unless in particular cases the description gives the words a special meaning by explicit definition. If a claim covers subject matter outside the scope of the described invention, it should be objected to for failing to satisfy the provisions of section 60 of the Patent Rules.
Date modified: March 1998
When an application includes claims containing a specific limitation with respect to operating conditions, which limitation falls within a broader range described, no objection is made to the narrow claim solely on the grounds that it is not specifically shown in the description or that the description does not indicate the significance of the described range. For example, an application may describe a process carried out within certain temperature limits, e.g. between 500°C and 800°C. No objection is made if some claims are directed to the process carried out between 500°C and 800°C and others to the process carried out at a temperature falling within a smaller range within the described range, e.g. between 650°C and 700°C. However, should the broad claim fall in view of prior art, the narrower claim would also fall unless it can be shown that by restricting the process to the narrower range, a new and unobvious result is obtained.
Date modified: October 2019
Section 63 of the Patent Rules permits a claim to refer to one or more other claims, in order to define an invention more narrowly by adding further characteristics to those already present in the claims to which reference is made. Such a claim is designated as a dependent claim.
Claims are also permitted to refer to other claims or parts of claims of the same or of another category, in order to avoid repeating lengthy definitions already given and to simplify claiming, provided they do not become ambiguous as a result of such dependency, thereby contravening section 27(4) of the Patent Act. Such claims however are not dependent claims and section 63 of the Patent Rules does not apply. The patentability of the claim referred to does not necessarily imply the patentability of the dependent claim containing the reference. The following example indicates the form of claiming that is acceptable.
Claim 1: A product comprising composition A.
Claim 2: A process for the production of the composition defined in claim 1 comprising reacting B with C.
An objection is made whenever there is uncertainty as to which part of a preceding claim reference is made or whenever a dependent claim of one category, such as a process, contains by reference so many limitations of another category, such as a product, that it becomes difficult to determine which category the claim covers.
A dependent claim usually refers to other claims in its preamble. In view of subsection 63(1) of the Patent Rules, a dependent claim must state the additional features claimed. According to subsection 63(4) of the Patent Rules, a dependent claim is understood as including all the limitations inherent in the particular claim or claims in relation to which it is considered. When a claim refers to other claims it must only refer to preceding claims and it must do so to by number. A claim that refers to more than one claim must refer to those claims in the alternative only (subsection 63(3) of the Patent Rules). A common phrase that can be used to refer to claims in the alternative is “…according to any one of claims…”.
|
Examples: |
|
|
Claim 1: |
The process of reacting A with B in the presence of a catalyst. (acceptable) |
|
Claim 2: |
The process of reacting A with B in the presence of a metal containing catalyst. (acceptable) |
|
Claim 3: |
The process of claim 2 in which the catalyst contains iron. (acceptable) |
|
Claim 4: |
The process of claim 3 in which the catalyst contains copper. (acceptable) |
|
Claim 5: |
The process of claim 1, 2, 3, or 4 in which the catalyst contains zinc. (acceptable) |
|
Claim 6: |
The process according to any one of claims 1 to 5 in which the catalyst contains cobalt. (acceptable) |
|
Claim 7: |
The process according to any of the above claims in which the catalyst is supported on an inert carrier. (not acceptable) |
|
Claim 8: |
The process of claim 5 in which the catalyst is supported on an inert carrier. (acceptable) |
|
Claim 9: |
The process of claim 6 in which the catalyst is supported on an inert carrier. (acceptable) |
|
Claim 10: |
The process of claim 8 or 9 in which the inert carrier is a silica. (acceptable) |
|
Claim 11: |
The process of claims 3 and 4 in which the catalyst contains manganese. (not acceptable) |
|
In the examples given above, no objection would be taken to claims 1-6 and 8-10 in view of the provisions of section 63 of the Patent Rules. In contrast, claim 7 which does not refer to the preceding claims by number, would, consequently, violate subsection 63(1) of the Patent Rules and would therefore be objected to. Claim 11 does not refer to claims 3 and 4 in the alternative and would be identified as a defect under subsection 63(3) of the Patent Rules. |
|
Date modified: October 2019
In order to comply with subsection 50(1) of the Patent Rules, the pages of the claims must be numbered consecutively and run continuously from the last page of the description, such that the pages of the specification as a whole are numbered consecutively.
Date modified: October 2019
In accordance with section 193 of the Patent Rules, for applications filed on or after October 1, 1996, but prior to October 30, 2019 (i.e. category 3 applications), the applicant may comply with the requirements of subsection 73(1) of the former Patent Rules which specified that the pages of the description and the claims shall be numbered consecutively.
Date modified: March 1998
A combination is a union of elements or process steps co-operating to produce a unitary and practical result that is not the sum of the known characteristics of the elements or steps.
A patentable combination is one in which the elements or steps cooperate in an unexpected manner or cooperate in a known way to give an unobvious result or effect. If all the requirements of the Patent Act and Rules are met, a claim to such a combination can be allowed.
A subcombination is part of a combination. It may be a single element or step of the combination or may, itself, be a combination.
Date modified: March 1998
Claims must not exceed the scope of the invention by going further than the protection to which the inventor is entitled. Generally, an inventor is entitled to claim the invention, be it apparatus, product or method and its immediate and cooperating environment. For example, claims to a new accelerator pump and the carburetor containing it are permitted. Also, claims to a new type of radio tube grid may be permitted with claims to the tube containing the grid. But claims to a new pump in a carburetor which is attached to an engine or claims to a radio receiver accommodating a tube having a new grid would be objected to unless the overall combination produced new and unexpected results, amounting to further invention, that may require restriction under section 36 of the Patent Act.
Date modified: March 1998
The information in this subsection has been moved to subsection 18.02.04 of this manual.
Date modified: March 1998
In product claims, the product may be defined in three ways:
A claim that defines a product by a mixture of two or three of these forms is also possible.
The most explicit and definite form of claims for a product defines the product by structure. Since, under subsection 27(4) of the Patent Act, the applicant is required to distinguish any new product from all other products by claiming it distinctly and explicitly, the structure, if known, should be given in the claim.
Date modified: March 1998
A product-by-process claim defines the claimed product wholly or partly in terms of the process used to produce the product. The process limitations may be included within the product claim itself or the whole claim may be made dependent upon another claim directed to the process. The following examples show the two possible forms:
The use of past participle adjectives, such as welded, bent, molded or coated, is not construed as changing a product claim into a product-by-process claim.
A product-by-process claim, where permitted, must define the product explicitly and distinguish it from all other products. Hence, products that are already known may not be claimed by making them dependent on a new process (Hoffman-La Roche v. Commissioner of Patents 23 C.P.R. 1).
A product-by-process claim must be directed to the final product of the process claim upon which the product claim is made dependent.
Date modified: March 1998
A "means" claim is one in which at least part of an invention is defined as a means or mechanism for performing an act, instead of reciting the element that performs the action.
Invention may exist in a new combination of old means (Lightning Fastener v. Colonial Fastener 51 RPC 349; Martin and Biro Swan v. H. Millwood 1956 RPC 125). Claims composed of more than one statement of old means are allowable, without defining structure, if there is invention in the new combination.
If a claim is composed of a single statement of means, it is objected to for being indefinite and contrary to subsection 27(4) of the Patent Act. The report of the examiner should indicate in detail why the claim contravenes subsection 27(4) of the Patent Act. It may, for example, be directed to the result desired rather than to the combination developed and illustrated to achieve that result.
A claim is also objected to if it contains a broad means statement at the point of invention, i.e., a statement that distinguishes the claim from the prior art, but which is so broad that it embraces all possible means without qualification for solving the problem facing the inventor and is in effect no more than a restatement of the problem or desired result.
Examples:
An application describes a sanding device that may be used in a direct-drive mode for removing stock from a work piece at a rapid rate or in an orbital mode for removing stock at a much slower rate to provide a smooth finish. The invention lies in the combined use of a known one-way clutch and a known reversible motor in an otherwise conventional rotary sander. Under prior art conditions, either two sanders were used or an attachment was employed to convert a device from a direct-drive sander to an orbital sander.
Claim (i) Means for operating a sanding device in either a direct-drive mode or an orbital mode.
This claim would be objected to under section 27(4) of the Patent Act. The applicant should claim a sander having the combination of a one-way clutch with a reversible motor.
Claim (ii) A surface-finishing device comprising a drive shaft, a driven element connected to receive drive from the drive shaft, a driven shaft mounted for rotation in said driven element about an axis eccentric to the axis of the drive shaft, means connecting the driven shaft to the driven element, a surface-finishing tool connected to be driven by the driven shaft, and automatic means for selectively connecting the surface-finishing tool directly to the drive shaft, or allowing said tool to rotate freely in an orbital path about the drive shaft axis.
This claim would be objected to under section 27(4) of the Patent Act for merely restating the desired result.
Claim (iii) A surface-finishing device comprising a drive shaft, a driven element connected to receive drive from the drive shaft, a driven shaft mounted for rotation in said driven element about an axis eccentric to the axis of the drive shaft, one-way clutch means connecting the driven shaft to the driven element, a surface-finishing tool connected to be driven by the driven shaft, and means for selectively driving the drive shaft in one direction or in an opposite direction.
This claim would be accepted as a novel combination of known means giving a new and unexpected result.
Date modified: March 1998
The Patent Office accepts process, method, method of use and use claims as explained under the following subheadings.
Date modified: March 1998
A method is the series of steps to be followed either alone or in conjunction within a process in order to achieve a desired result. A method should be distinguished from a process, which includes the method and the substances to which it is applied. The overall process may be new even though the method is old.
A claim to a process which consists of applying a known method to chemically react known substances is patentable, providing the method has never before been applied to these substances and results in new, useful and unobvious products. (Ciba Ltd. v. Commissioner of Patents 27 C.P.R. 82; 30 C.P.R. 135).
Date modified: March 1998
When a claim to a compound has been found allowable in an application, then a claim to a method of use of that compound or a claim to the use of that compound is also allowable in the same application. When a claim to a compound has been found allowable to the inventor in one application, then claims in a different application of the same inventor to a use of that compound or methods of using that compound which are obvious from the utility disclosed for the compound, and upon which utility the patentability of the compound was predicated, are not allowed.
When a compound has been patented previously or is in the public domain, claims directed to the obvious use of this compound should be objected to for lacking patentable subject matter. Claims directed to a new and unobvious use of the same compound are allowable. Likewise, claims directed to a method of using the compound for a new unobvious purpose are allowable. Furthermore, when an invention is directed to a novel and unobvious use of a known compound, claims to this known compound with the further recitation of a novel use are allowable (re application for patent of Wayne State University 22 C.P.R. (3d) 407).
When a device or machine is only a new instrument for carrying out an old method, only the device or machine can be patented. Since the utility of a device or machine is obvious from the description of the device or machine, the patentability of a method using such device or machine is determined by the state or the art.
Guidelines for method of use claims
Example: Method of treating the symptoms of cognitive decline in a patient comprising administering to a patient an effective amount of compound X wherein said compound is used as a cholinergetic agent. (rejected)
Example: Method for enhancing the dressed carcass weight of meat-producing animals by increasing lean meat deposition and improving the lean meat to fat ratio comprising administering to said animals, before slaughter, either orally or parenterally, an effective amount of a compound X. (accepted)
Example: Method of using compound X as an intermediate to prepare compound Y wherein compound X is reduced by hydroboration or catalytic hydrogenation. (accepted)
Example: Method of controlling agricultural bacteria which comprises incorporating into the locus to be treated an effective amount of compound X wherein said compound is used as a bacterial agent. (accepted)
Example: Compound X for the use as an insecticide wherein said compound is applied to the locus of a tree trunk, (accepted).
Example: Compound Y for the treatment of viruses wherein said compound is administered to a patient intravenously, (not accepted because it contains a method of medical treatment).
Examples:
Use of compound X as a herbicide. (accepted)
Use of compound X as a herbicide wherein an effective amount of the compound X is incorporated into the locus to be treated. (accepted)
Use of compound Y as an antiarrhythmic agent. (accepted)
Use of compound Y as an antiarrhythmic agent wherein an effective amount of the compound Y is administered to a patient. (not accepted). The addition of the "wherein" clause makes the use a method of medical treatment.
Use of machine Z for cutting. (accepted)
Use of machine Z for cutting wherein ... (accepted)
Date modified: March 1998
In chemical cases, a claim directed to a genus expressed as a group consisting of certain specified materials is allowable (Ex parte Markush 1925, 340 U.S.O.G. 839) provided it is clear from the known nature of the alternative materials or from the prior art that the materials in the group possess at least one property in common which is mainly responsible for their function in the claimed relationship. Therefore, a Markush claim will generally be construed with a generic expression covering a group of two or more different materials (elements, radicals, compounds) as illustrated in the following examples:
A solvent selected from the group consisting of alcohol, ether and acetone...
A strip of a conductive metal selected from the group consisting of copper, silver and aluminium...
Occasionally, the Markush format may be used in claims directed to subject matter in the mechanical or electrical fields in a manner such as that illustrated in the example below:
A means for attaching a wall panel to a framework wherein the attaching means is selected from group consisting of nails, rivets and screws...
Date modified: March 1998
The information in this subsection has been moved to section 18.07 of this manual.
Date modified: March 1998
The following decisions of the courts are of importance in considering the subject matter of this chapter:
|
claims construction |
|
|
Minerals Separation v Noranda |
12 CPR 99 1950; 69 RPC 81 1952 |
|
O'Cedar v Mallory Hardware |
ExCR 299 1956 |
|
McPhar v Sharpe |
35 CPR 105 1960 |
|
Metalliflex v Wienenberger |
35 CPR 49 1961; SCR 117 1961 |
|
Lovell v Beatty |
41 CPR 18 1962 |
|
Burton Parsons v Hewlett |
1 SCR 555 1976 |
|
Xerox v IBM |
33 CPR (2d) 24 1977 |
|
Cutter v Baxter Travenol |
68 CPR (3d) 179 1983 |
|
Johnston Controls v Varta |
80 CPR (2d) 1 1984 |
|
Reading & Bates v Baker |
18 CPR (3d) 181 1987 |
|
AT&T Tech v Mitel |
26 CPR (3d) 238 1989 |
|
Energy v Boissonneault |
30 CPR (3d) 420 1990 |
|
Lubrizol v Imperial Oil |
33 CPR (3d) 11 1990; 45 CPR (3d) 449 1992 |
|
Computalog v Comtech |
32 CPR (3d) 289 1990; 44 CPR (3d) 77 1992 |
|
Procter & Gamble v Kimberly |
40 CPR (3d) 1 1991 |
|
Wellcome v Apotex |
39 CPR (3d) 289 1991 |
|
TRW Inc v Walbar |
39 CPR (3d) 176 1991 |
|
Martinray v Fabricants |
14 CPR (3d) 1 1991 |
|
Reliance v Northern Tel |
47 CPR (3d) 55 1993 |
|
Airseal v M&I Heat |
53 CPR (3d) 259 1993 |
|
Dableh v Ont Hydro |
50 CPR (3d) 290 1993 |
|
Unilever v Procter & Gamble |
47 CPR (3d) 479 1993; 61 CPR (3d) 499 1995 |
|
Nekoosa v AMCA Int |
56 CPR (3d) 470 1994 |
|
Anderson v Machineries |
58 CPR (3d) 449 1994 |
|
Pallmann v CAE |
62 CPR (3d) 26 1995 |
|
Hi-Quail v Rea's Welding |
55 CPR (3d) 224 1994 |
|
Feherguard v Rocky's |
53 CPR (3d) 417 1994; 60 CPR (3d) 512 1995 |
|
Cochlear v Coseum |
64 CPR (3d) 10 1995 |
|
Pallmann v CAE |
62 CPR (3d) 26 1995 |
|
Almecon v Nutron |
65 CPR (3d) 417 1996 |
|
positive recitation |
|
|
Minerals Separation v Noranda |
12 CPR 99 1950; 69 RPC 81 1952 |
|
Burton Parsons v Hewlett |
1 SCR 555 1976 |
|
Eli Lilly v O'Hara |
20 CPR (3d) 342 1988; 26 CPR (3d) 1 1989 |
|
Hi-Quail v Rea's Welding |
55 CPR (3d) 224 1994 |
|
Pallmann v CAE |
62 CPR (3d) 26 1995 |
|
Antecedents |
|
|
Mobil Oil v Hercules |
57 CPR (3d) 488 1994; 63 CPR (3d) 473 1995 |
|
Preamble |
|
|
Re: Lelke |
72 CPR (2d) 139 1981 |
|
Shell Oil v Comm of Pat |
2 SCR 536 1982 |
|
Rucker V Gavels Vulcanizing |
7 CPR (3d) 294 1985 |
|
Permacon v Enterprises |
19 CPR (3d) 378 1987 |
|
Re: Neuro Med Inc |
28 CPR (3d) 281 1988 |
|
Computalog v Comtech |
44 CPR (3d) 77 1992 |
|
explicit, distinct v ambiguous/several interpretations |
|
|
Rohm & Haas v Comm of Patents |
30 CPR 113 1959 |
|
Xerox v IBM |
33 CPR (2d) 24 1977 |
|
Monsanto v Comm of Pat |
42 CPR (2d) 161 1979; 2 SCR 1108 1979 |
|
Ciba Geigy v Comm of Pat |
65 CPR (3d) 73 1982 |
|
Pioneer Hi-Bred v Com of Pat |
14 CPR (3d) 491 1987; 25 CPR (3d) 257 1987 |
|
Reliance v Northern Tel |
28 CPR (3d) 397 1989; 44 CPR (3d) 161 1992; 47 CPR (3d) 55 1993 |
|
Risi Stone v Groupe Peracon |
29 CPR (3d) 243 1990; 65 CPR (3d) 2 1995 |
|
Allied v Du Pont |
52 CPR (3d) 351 1993; 50 CPR (3d) 1 1993 |
|
Mobil Oil v Hercules |
57 CPR (3d) 488 1994; 63 CPR (3d) 473 1995 |
|
insufficient/sufficient/essential elements |
|
|
BVD Co V Canadian Celanese |
ExCR 139 1936; SCR 221 1937 |
|
Minerals Separation v Noranda |
12 CPR 99 1947; 15 CPR 133 1952 |
|
Curl Master v Atlas Brush |
SCR 514 1967 |
|
Burton Parsons v Hewlett |
1 SCR 555 1976 |
|
Re: Farbwerke Hoechst |
13 CPR (3d) 212 1980 |
|
Ciba Geigy v Comm of Pat |
65 CPR (3d) 73 1982 |
|
Consolboard v MacMillan |
56 CPR (2d) 145 1981; 1 SCR 504 1981 |
|
Ductmate v Exanno |
2 CPR (3d) 289 1984 |
|
Amfac Foods v Irving Pulp |
12 CPR (3d) 193 1986 |
|
Crila Plastics v Ninety Eight |
10 CPR (3d) 226 1986; 18 CPR (3d) 1 1987 |
|
Reliance v Northern Tel |
28 CPR (3d) 397 1989; 44 CPR (3d) 161 1992; 47 CPR (3d) 55 1993 |
|
TRW Inc v Walbar |
39 CPR (3d) 176 1991 |
|
Atlas v CIL |
41 CPR (3d) 348 1992 |
|
Airseal v M&I Heat |
53 CPR (3d) 259 1993 |
|
Mobil Oil v Hercules |
57 CPR (3d) 488 1994; 63 CPR (3d) 473 1995 |
|
Feherguard v Rocky's |
53 CPR (3d) 417 1994; 60 CPR (3d) 512 1995 |
|
Operability |
|
|
Union Carbide v Trans Canadian |
ExCR 884 1965 |
|
Minerals Separation v Noranda |
12 CPR 99 1950; 69 RPC 81 1952 |
|
Gilbert (Gillcross) v Sandoz |
64 CPR 14 1970; SCR 1336 1974 |
|
Burton Parsons v Hewlett |
1 SCR 555 1976 |
|
Sandvick v Windsor |
8 CPR (3d) 433 1986 |
|
Mahurkar v Vas-Cath |
18 CPR (3d) 417 1988 |
|
Wellcome v Apotex |
39 CPR (3d) 289 1991 |
|
TRW Inc v Walbar |
39 CPR (3d) 176 1991 |
|
Feherguard v Rocky's |
53 CPR (3d) 417 1994; 60 CPR (3d) 512 1995 |
|
Mobil Oil v Hercules |
57 CPR (3d) 488 1994; 63 CPR (3d) 473 1995 |
|
Broad |
|
|
BVD Co V Canadian Celanese |
ExCR 139 1936; SCR 221 1937 |
|
Trubenizing v John Forsyth |
2 CPR 1 1943 |
|
O'Cedar v Mallory Hardware |
ExCR 299 1956 |
|
Lovell v Beatty |
41 CPR 18 1962 |
|
Boehringer v Bell-Craig |
39 CPR 201 1962 |
|
Union Carbide v Trans Canadian |
ExCR 884 1965 |
|
Hoechst v Gilbert |
SCR 189 1966 |
|
Gilbert v Sandoz |
64 CPR 14 1970 |
|
Burton Parsons v Hewlett |
1 SCR 555 1976 |
|
Monsanto v Comm of Pat |
42 CPR (2d) 161 1979; 2 SCR 1108 1979 |
|
Re: American Home Products |
55 CPR (2d) 238 1980 |
|
Re: Farbwerke Hoechst |
13 CPR (3d) 212 1980 |
|
Cutter v Baxter Travenol |
50 CPR (2d) 163 1980; 68 CPR (3d) 179 1983 |
|
Johnston Controls v Varta |
80 CPR (2d) 1 1984 |
|
Sandvick v Windsor |
8 CPR (3d) 433 1986 |
|
Amfac Foods v Irving Pulp |
12 CPR (3d) 193 1986 |
|
Cabot Corp v 318602 Ont |
20 CPR (3d) 132 1988 |
|
Mahurkar v Vas-Cath |
18 CPR (3d) 417 1988 |
|
Reliance v Northern Tel |
28 CPR (3d) 397 1989; 44 CPR (3d) 161 1992; 47 CPR (3d) 55 1993; 55 CPR (3d) 299 1994 |
|
Risi Stone v Groupe Peracon |
29 CPR (3d) 243 1990 |
|
Lubrizol v Imperial Oil |
33 CPR (3d) 1 1990; 45 CPR (3d) 449 1992 |
|
Wellcome v Apotex |
39 CPR (3d) 289 1991 |
|
Dableh v Ont Hydro |
50 CPR (3d) 290 1993 |
|
Unilever v Procter & Gamble |
47 CPR (3d) 479 1993; 61 CPR (3d) 499 1995 |
|
Mobil Oil v Hercules |
57 CPR (3d) 488 1994; 63 CPR (3d) 473 1995 |
|
Nekoosa v AMCA Int |
56 CPR (3d) 470 1994 |
|
Pallmann v CAE |
62 CPR (3d) 26 1995 |
|
Almecon v Nutron |
65 CPR (3d) 417 1996 |
|
selection/improvement |
|
|
Sherbrooke v Hydrolic |
Ex CR 114 1927 |
|
Bergeon v De Kermor |
Ex CR 181 1927 |
|
Western Electric v Bell |
Ex CR 213 1929 |
|
Wandscheer v Sicard |
SCR 1 1948 |
|
K v Uhleman Optical |
Ex CR 142 1950; 1 SCR 143 1952 |
|
O'Cedar v Mallory Hardware |
Ex CR 299 1956 |
|
Ciba Geigy v Comm of Pat |
27 CPR 82 1957; 30 CPR 135 1959 |
|
aggregation/combination |
|
|
Lightning Fastener v Colonial |
Ex CR 89 1932; SCR 63 1933; 51 RPC 349 1934 |
|
Crosley Radio v CGE |
SCR 551 1936 |
|
Lanlois v Roy |
Ex CR 197 1941 |
|
Lester v Comm of Pat |
Ex CR 603 1946 |
|
Wandscheer v Sicard |
Ex CR 112 1946; SCR 1 1948 |
|
R v Uhleman Optical |
Ex CR 142 1950; 1 SCR 143 1952 |
|
Defrees v Dominion Auto |
Ex CR 331 1963 |
|
Barton v Radiator Specialty |
44 CPR 1 1965 |
|
Gibney v Ford |
2 Ex CR 279 1972 |
|
Rubbermaid v Tucker Plastics |
8 CPR (2d) 6 1972 |
|
Agripat v Comm of Patents |
52 CPR (2d) 229 1977 |
|
Domtar v MacMillan |
33 CPR (2d) 182 1977 |
|
Xerox v IBM |
33 CPR (2d) 24 1977 |
|
Ductmate v Exanno |
2 CPR (3d) 289 1984 |
|
Windsurfing v Triatlantic |
3 CPR (3d) 95 1984 |
|
Hy Kramer v Lindsay |
9 CPR (3d) 297 1986 |
|
Crila Plastics v Ninety Eight |
10 CPR (3d) 226 1986; 18 CPR (3d) 1 1987 |
|
Hoffman-La Roche v Apotex |
15 CPR (3d) 217 1987; 24 CPR (3d) 289 1989 |
|
Standal v Swecan |
28 CPR (3d) 261 1989 |
|
Imperial Tobacco v Rothmans |
47 CPR (3d) 188 1993 |
Date modified: November 2017
The protection offered by the Patent Act extends to many but not all types of human endeavour; those types to which it applies are called “statutory”.
Section 2 of the Patent Act defines an invention as:
any new and useful art, process, machine, manufacture or composition of matter, or any new and useful improvement in any art, process, machine, manufacture or composition of matter.
In order to be considered statutory, the subject-matter for which protection is sought must fall within one of these categories of subject-matter defined in section 2 of the Patent Act. The requirement that an invention be statutory can be framed in terms of asking whether or not the invention is proper “subject-matter” for a patent.
Date modified: November 2017
The term “art”, for the purposes of the Patent Act, pertains to the application of knowledge to effect a desired result.[122] To be statutory, an “art” must be what the courts have termed a “useful art”[123] and a “manual or productive art.”[124] An art must be the practical application of knowledge,[125] and must therefore be defined in a manner that gives practical effect to the knowledge. An art, therefore, is typically claimed as either a use or a method.
A use claim typically sets out a manner or mode of employing something in order to accomplish a particular result without prescribing in detail how the result is to be achieved. For example, a use claim might take the form “Use of a heat source to boil water.”
[See section 16.10.02 for further guidance on use claims.]
A “method” claim also sets out a mode or manner of accomplishing a certain result but includes one or more particular steps required to achieve the result. For example, a method claim might take the form of “A method of heating water comprising the steps of pouring two cups of water into a stainless steel container, placing the container on a heat source, and heating the water until the water temperature reaches 100 degrees Celsius.”
Whether or not a method is statutory is not determined by whether or not it produces a statutory product.
Date modified: November 2017
A “process” implies the application of a method to a material or materials.[126] A process can be considered to be a mode or method of operation by which a result or effect is produced by physical or chemical action, by the operation or application of some element or power of nature; or of the application of one substance to another. As with methods, whether or not a process is statutory is not determined by whether or not it produces a statutory product.
Date modified: November 2017
A “machine” is the mechanical and/or physical embodiment of any function or mode of operation designed to accomplish a particular effect, wherein the parts of the machine cooperate to accomplish the effect. A machine can be considered to be “any device that transmits a force or directs its application”
, or “a device that enables energy from one source to be modified and transmitted as energy in a different form or for a different purpose”
.[127] A machine may be claimed as a device, as an apparatus, or a system, for example.
Date modified: November 2017
A “manufacture” has been broadly defined as “a non-living mechanistic product or process” and as being the process of making (by hand, by machine, industrially, by mass production) technical articles or material (in modern use on a large scale) by the application of physical labour or mechanical power; or the article or material made by such a process.[128]
Date modified: November 2017
A “composition of matter” refers to physical and/or chemical substances, compounds and compositions, and includes combinations of ingredients, whether combined as a chemical union or physical mixture. The term “matter” implies that the ingredients must be perceptible in space and have mechanical mass. In Harvard College v. Canada (Commissioner of Patents), the Supreme Court noted that the scope of this category must be limited in some way, else the categories of "machine" and "manufacture" would be made redundant.[129]
Date modified: November 2017
An invention is a solution to a practical problem. In order to solve a practical problem, the solution must be something with physical existence, or something that manifests a discernible effect or change[130] and, hence, that will itself enable a person skilled in the art to obtain the intended result or benefit. Such a form is referred to herein as a "practical form" or a "practicable form".
A disembodied idea, concept or discovery that underlies or leads to an invention is not itself patentable; in order to be patentable it must be incorporated in a practical form. A mere idea or intellectual concept, no matter how well it may have been worked out and structured in the mind, is disembodied and is not something with physical existence, or something that manifests a discernible effect or change.. In Shell Oil Co. v. Commissioner of Patents the Supreme Court noted that “a disembodied idea is not per se patentable. But it will be patentable if it has a method of practical application.”[131] In Riello Canada Inc. v. Lambert, the court cited with approval comments from Reynolds v. Herbert Smith & Co., Ltd., which noted that "the idea that leads to an invention is [...] no part of the invention. The idea, or the recognition of the want, stimulates the inventor to do something else. It is the something further which he does which is the invention"
and similarly that "discovery adds to the amount of human knowledge, but it does so only by lifting the veil and disclosing something which before had been unseen or dimly seen. Invention also adds to human knowledge, but not merely by disclosing something. Invention necessarily involves also the suggestion of an act to be done, and it must be an act which results in a new product, or a new result, or a new process, or a new combination for producing an old product or an old result"
.[132]
Date modified: November 2017
It is apparent from the definition of invention in section 2 of the Patent Act that not everything can be patented. With respect to section 2, the Supreme Court noted in Harvard College v. Canada that “[b]y choosing to define invention in this way, Parliament signaled a clear intention to include certain subject-matter as patentable and to exclude other subject-matter as being outside the confines of the Act.”
[133]
The following sections set out various statutory and jurisprudential proscriptions to the scope of patentable subject-matter under section 2 of the Patent Act.
Date modified: November 2017
Subsection 27(8) of the Patent Act states:
No patent shall be granted for any mere scientific principle or abstract theorem.
This subsection has been interpreted by the courts as excluding from patentability (inter alia) mathematical formulae[134], natural phenomena and laws of nature.
The exclusions of this subsection apply when an attempt is made to claim the excluded subject-matter in a general sense, but not when a scientific principle, law of nature or mathematical formula is relied upon in operating a practical form of an invention.
The Patent Office considers that mere scientific principles and abstract theorems do not constitute an invention within the meaning of section 2 of the Patent Act. Accordingly, claims that are found to be directed to scientific principles or abstract theorems will be identified as defective under both subsection 27(8) and section 2 of the Patent Act.
Date modified: November 2017
A method or process of surgery or therapy on living humans or animals is not considered to be within the scope of the meaning of invention as set out in section 2 of the Patent Act.
A detailed consideration of medical and surgical methods can be found in section 23.03.
Date modified: November 2017
The Supreme Court of Canada has determined that higher life forms are excluded from patentability by virtue of their not being either manufactures or compositions of matter within the definition of invention as set out in section 2 of the Patent Act.[135]
A detailed consideration of higher life forms can be found in section 23.02.01.
Date modified: November 2017
Forms of energy such as regions of the electromagnetic spectrum, electric currents and explosions are not considered to be subject-matter within the scope of the meaning of invention as set out in section 2 of the Patent Act.
Forms of energy are not considered to be manufactures or compositions of matter in the sense intended by the Patent Act. Electromagnetic and acoustic signals are also considered to be forms of energy and do not contain matter even though the signal may be transmitted through a physical medium. Thus, claims to electromagnetic and acoustic signals do not constitute statutory subject-matter within the meaning of section 2 of the Patent Act.
More particularly, an electromagnetic or acoustic signal is not considered to be an art (i.e. not a method or a use per se) nor a process (i.e. not a mode or method of operation by which a result or effect is produced by physical or chemical action; by the operation of application of some element of power of nature; or by the application of one substance to another). Neither is an electromagnetic or acoustic signal a machine, as it is not the mechanical embodiment of any function or mode of operation designed to accomplish a particular effect, nor is it a composition of matter, as it is not a chemical compound, composition or substance. An electromagnetic or acoustic signal is taken not to be itself a material product and is therefore, also not a manufacture.
Date modified: November 2017
Features of an invention that have a purely intellectual or aesthetic significance are considered, in a practical sense, not to affect the functioning of the invention. Such features cannot change the manner in which the practical form of an invention operates to solve the problem for which it is the solution.
Where a claim appears to be directed to subject-matter having solely intellectual or aesthetic significance, the claim is defective under section 2 of the Patent Act.[136]
Where an invention requires a practical problem to be solved in order to enable a result or effect having solely intellectual or aesthetic significance, the patentability of the invention is not impacted by the fact its purpose is to produce a non-statutory result or effect.[137] In such cases, the practical form of the invention does not lie solely in its intellectual or aesthetic significance as the solution to the practical problem gives rise to a new functionality.
Date modified: November 2017
Printed matter that has purely intellectual or aesthetic significance, such as a literary work, is excluded from patentability for the reasons outlined in 17.03.05. However, where printed matter provides a new functionality to the substrate on which it is printed, a claim to this subject-matter may be considered statutory. For the printed matter and the substrate to be considered to be a practical form of an invention, they must solve a practical problem related to the use of the printed matter in general, and not be based solely on the intellectual or aesthetic content of the printed matter itself.
By way of example, each of the following has been found by the Commissioner of Patents as being patentable: a textile material bearing markings to enable greater precision during a manufacturing procedure,[138] a newspaper layout in which white space is left to facilitate reading when the paper is folded, a layout of text on a series of pages to facilitate a bookbinding process, and a layout of text on a ticket which permits the ticket to be divided either horizontally or vertically while ensuring all information will appear on both halves.[139]
In each of the foregoing the printed matter provided a new mechanical functionality to the combination; the actual content of the printed matter was not the basis of the invention. Where printed matter has only intellectual or aesthetic significance, it may conveniently be referred to as “non-functional descriptive matter”.
The term “printed matter” should not be restricted to traditional ink-on-paper printing but may include any means of displaying information.
Example:
An application describes a new scratch-off lottery ticket wherein the scratchable areas are arranged in a maze-pattern, wherein the user must scratch one cell at a time to determine if they can move their way to the end of the maze.
Claim:
A scratch-off lottery ticket comprising a pattern or a plurality of intersecting pathways that define a maze, said pathways divided into individual cells, each cell including an indicator of direction and each cell being covered by an opaque scratchable material, wherein if the indicators of direction define a path from a first cell of the maze to a final cell of the maze, the lottery ticket is a “winning ticket.”
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is considered to be a person who is skilled in the design of scratch-off lottery tickets; the POSITA is also knowledgeable in the field of marketing.
Common General Knowledge (CGK) of the POSITA
The POSITA would consider that substrates on which information is concealed under opaque scratchable material are CGK. The use of such substrates in the art of scratch-off lottery tickets having various game scenarios would also be considered to be CGK.
The Problem
The POSITA, having read the specification and in light of their CGK, would consider that the problem addressed by the claimed invention was to provide a variation on scratchable lottery tickets.
The Solution
The solution to the problem is the provision of the pattern or the plurality of intersecting pathways that define a maze.
What are the essential elements?
The essential element (i.e. the element that provides the solution to the problem) is the pattern or the plurality of intersecting pathways that define a maze.
Is the claim statutory?
This essential element provides no new functionality to the substrate on which it is printed; it is merely printed matter that has solely intellectual or aesthetic significance. The claim is directed to non-statutory subject-matter and is therefore non-compliant with section 2 of the Patent Act.
Date modified: November 2017
A fine art has been described as “that having intellectual meaning or aesthetic appeal alone”
.[140] Fine arts are therefore not patentable subject-matter.[141] The term is understood to include activities such as exercising, dancing, acting, writing, teaching, hair dressing, cosmetology, flower arranging, painting pictures and playing musical instruments. Generally, any product derived from a fine art will also be non-statutory.
Fine arts and the products thereof are not a practical form of an invention since they do not solve any practical problem. Typically, the features that distinguish a product produced by a fine art will have purely intellectual or aesthetic significance.
The exclusion from patentability of fine arts does not extend to inventive materials and instruments used in practising a fine art. For example, while an artistic method of painting a picture and the resultant picture are non-statutory, an inventive easel for holding a canvas would be patentable. Similarly, the paints, paint-brushes etc., used in conjunction with the fine art – but not derived from the fine art as the picture is – may be considered to be statutory subject-matter.
Date modified: November 2017
A scheme, plan, or rule for performing an operation, achieving a result or controlling a method,[142] and a process that is exclusively a series of mental steps[143] (e.g., performing calculations; manipulating data or information to produce data or information having a different purely intellectual meaning or aesthetic significance) are disembodied (abstract) and are not a practical form of an invention regardless of reproducibility.
Date modified: November 2017
A manner of playing a game or sport does not solve a practical problem, and a method for playing a game is therefore non-statutory. This is so whether the claimed method is distinguished on the basis of specific rules governing play[144] or in terms of actions to be taken to achieve specific game-related results.
Tools made use of in the playing of a game may themselves be patentable (e.g., a specifically designed table or playing piece or a game board with a particular mechanical function, or combination of such that is patentable on its own merits).
Date modified: June 2016
The requirement that an invention be novel finds its basis in the definition of invention in section 2 of the Patent Act – “any new [...] art, process, machine, manufacture or composition of matter or any new and useful improvement in any art, process, machine, manufacture or composition of matter”
.[145]
In order for an invention to be novel, it must be established that individual disclosures in the prior art do not anticipate the claimed invention.
Whether a given disclosure is considered to be prior art is governed by section 28.2 of the Patent Act. Although any public disclosure of information may be considered in principle, for practical reasons the assessment is almost exclusively performed on the basis of written disclosures.
Anticipation is assessed on a claim-by-claim basis by asking whether the prior disclosure, when understood by the person skilled in the art in light of their common general knowledge, provides both a description of the claimed invention (disclosure) and sufficient instructions to enable the invention to be practised (enablement).[146]
The comparison of the claimed invention to the prior disclosure is based on a comparison of the essential elements of the claim, properly construed, to the prior art.[147] Elements that are not required in order for the invention to solve the problem the inventors set out to address need not be disclosed in the anticipatory prior art. Furthermore, an invention is considered to have been previously described where the subject-matter previously disclosed would, if performed, infringe the later claim.[148]
A prior disclosure is considered to be enabling for the purpose of anticipation if the person skilled in the art, where necessary through trial and error experimentation that is neither inventive nor an undue burden, can operate the disclosed invention successfully.[149]
Date modified: October 2019
Section 28.2 of the Patent Act defines what disclosures may be considered for the purpose of assessing anticipation. In summary, this section establishes: a grace period of an application during which disclosures originating from the applicant are excluded as prior art; third party disclosures anywhere in the world before the application claim date as prior art; and the conditions respecting first-to-file when a co-pending Canadian application filed by a person other than the applicant is prior art.
Pursuant to section 163 of the Patent Rules, an international application (i.e. one filed under the Patent Cooperation Treaty) is not considered to be a Canadian application for the purposes of first-to-file anticipation (paragraphs 28.2(1)(c) and (d) of the Patent Act) unless it has entered the national phase. Subsection 155(1) of the Patent Rules provides that once an international application enters the national phase to become a PCT national phase application, it is considered to be an application filed in Canada.
In accordance with subsection 28.2(2) of the Patent Act, a Canadian application that is withdrawn before being opened to public inspection is considered, for the purposes of paragraphs 28.2(1)(c) and (d), never to have been filed. Consequently, such an application is not eligible as first-to-file prior art. Any Canadian application that has been opened to public inspection may be eligible as prior art under 28.2(1)(c) or (d)even where it has been withdrawn, abandoned or refused.
Date modified: October 2019
Paragraph 28.2(1)(a) of the Patent Act provides that
The subject-matter defined by a claim in an application for a patent in Canada (the “pending application)” must not have been disclosed (a) before the one-year period immediately preceding the filing date or, if the claim date is before that period, before the claim date by the applicant, or by a person who obtained knowledge, directly or indirectly, from the applicant, in such a manner that the subject-matter became available to the public in Canada or elsewhere[.]
This provision defines self-anticipation which occurs when a public disclosure made by the applicant or by a person who obtained their knowledge directly or indirectly from the applicant is used as prior art for the assessment of anticipation, but excludes this disclosure as prior art if it was made in the grace period.
The grace period is one year before the filing date of the application, unless the claim date is earlier than that period, in which case the grace period is the period between the claim date and the filing date [See 18.04].
Date modified: June 2016
Paragraph 28.2(1)(b) of the Patent Act provides that
The subject-matter defined by a claim in an application for a patent in Canada (the “pending application”) must not have been disclosed
(b) before the claim date by a person not mentioned in paragraph (a) in such a manner that the subject‑matter became available to the public in Canada or elsewhere[.]
This provision defines any public disclosure made by a third party (i.e. by a person other than the applicant or a person who obtained their knowledge directly or indirectly from the applicant) as prior art for the assessment of anticipation if it was made before the claim date [see 18.03].
Date modified: October 2019
Paragraph 28.2(1)(c) of the Patent Act provides that
The subject-matter defined by a claim in an application for a patent in Canada (the “pending application”) must not have been disclosed
(c) in an application for a patent that is filed in Canada by a person other than the applicant, and has a filing date that is before the claim date[.]
This provision exists to give effect to first-to-file considerations, and allows a Canadian patent application that was not open to public inspection as of the subject application’s claim date and which would consequently not be citable under paragraph 28.2(1)(b) of the Patent Act to nevertheless be considered for the purpose of anticipation. Note that the entire content of the earlier application is considered in assessing anticipation. The analysis is not limited by the matter claimed in the earlier application.
This provision defines a Canadian co-pending patent application, made by a third party, not open to public inspection as of the subject application’s claim date and having a filing date earlier than the pending application claim date as prior art for the assessment of anticipation of the pending application. Paragraphs 28.2(1)(c) and (d) effectively establish Canada’s first-to-file regime.
Where the applicability of a Canadian application as prior art under paragraph 28.2(1)(c) depends on the validity and extent of the priority claim of the application being examined, the examiner should obtain the priority document and verify whether the filing date of the priority date may be used as the claim date (see 18.03).
Date modified: October 2019
Paragraph 28.2(1)(d) of the Patent Act provides that
The subject-matter defined by a claim in an application for a patent in Canada (the “pending application”) must not have been disclosed
(d) in an application (the “co‑pending application”) for a patent that is filed in Canada by a person other than the applicant and has a filing date that is on or after the claim date if
(i) the co‑pending application is filed by
(A) a person who has, or whose agent, legal representative or predecessor in title has, previously regularly filed in or for Canada an application for a patent disclosing the subject‑matter defined by the claim, or
(B) a person who is entitled to protection under the terms of any treaty or convention relating to patents to which Canada is a party and who has, or whose agent, legal representative or predecessor in title has, previously regularly filed in or for any other country that by treaty, convention or law affords similar protection to citizens of Canada an application for a patent disclosing the subject‑matter defined by the claim,
(ii) the filing date of the previously regularly filed application is before the claim date of the pending application,
(iii) the filing date of the co‑pending application is within twelve months after the filing date of the previously regularly filed application, and
(iv) the applicant has, in respect of the co‑pending application, made a request for priority on the basis of the previously regularly filed application.
This provision expands the definition of prior art for the assessment of anticipation of a pending application to include Canadian co-pending applications not open to public inspection as of the subject application’s claim date and having a claim date earlier than the pending application claim date.
The provision requires that the claims of the co-pending Canadian application benefit from a priority date that precedes the claim date of the application being examined. The filing date, information relating to the priority request, and content of the priority document of the co-pending application should be reviewed by the examiner to ensure that it has met all requirements necessary to benefit from this earlier claim date (see 18.03). The assessment is based on the entirety of the information benefiting from the priority date, and is not further limited by the claims.
Date modified: June 2016
The test for anticipation, as set out by the Supreme Court in Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61, requires that a single disclosure both disclose and enable the claimed invention.[150] The approach taken by the person skilled in the art in reading and applying the prior art differs slightly when assessing the two parts of the test.
The first part of the test for anticipation asks whether a single prior teaching discloses the same invention that has been claimed in the application under consideration (or, where a claim encompasses several embodiments, of at least one operating embodiment of the claimed invention). In reading the prior disclosure to understand the matter it describes, the skilled person[151] is “taken to be trying to understand what the author of the description [in the prior patent] meant”
.[152] The prior disclosure is read in the same informed and purposive manner as the application itself, so as to fairly interpret its teachings,[153] as if being read by the person skilled in the art at the claim date of the claim under consideration.[154] The disclosure does not have to be an “exact description” of the claimed invention. The disclosure must be sufficient so that when read by a person skilled in the art willing to understand what is being said, it can be understood “without trial and error”.[155] Even if the prior disclosure uses quite different terms to describe its subject-matter, “if carrying out the directions contained in the prior inventor’s publication will inevitably result in something being made or done which [...] would constitute an infringement”
of a claim being examined, the prior disclosure describes the same invention.[156]
If the prior teaching does disclose the claimed invention, the next part of the test must be evaluated. That is, does the prior disclosure enable the disclosed invention to be operated without inventive effort or undue experimentation? [See Chapter 14 of this manual as it relates to disclosure]. At this stage, the person skilled in the art “is assumed to be willing to make trial and error experiments to get [the invention] to work”
.[157] Note that enablement does not mean that the earlier invention was actually put into practice, but simply that the earlier disclosure was sufficient to enable the person skilled in the art to build, operate or use the invention. If, on a fair and balanced reading of an earlier disclosure, it is unclear whether the disclosure is enabling of the claimed invention, the examiner must set forth the reasons for considering that the disclosure is, in fact, enabling. In contrast, where an applicant asserts that inventive effort or an undue burden would be required to operate an invention in view of an earlier disclosure, this should be supported by reasoned arguments and, as appropriate, by relevant facts.
While particular expressions of the test for anticipation have been provided by various Courts, a common thread is that the prior teaching has to anticipate “for [the] purpose of practical necessity”,[158] implying that the test for anticipation is based on practical considerations rather than theoretical ones.[159] The test has been described as asking whether the prior art document gives “information which for the purpose of practical utility is equal to that given by the subject”
application,[160] and similarly as asking whether the prior disclosure would allow the person skilled in the art to understand “and be able practically to apply the discovery without the necessity of making further experiments and gaining further information before the invention can be made useful”
.[161]
In many circumstances, the concept of “reverse infringement” can be used to assess anticipation.[162] Based on the principle that “what amounts to infringement, if posterior, should, as a general rule, amount to anticipation, if anterior”
,[163] anticipation by reverse infringement asks “if the earlier disclosure were to be put into practice, would it infringe the later claims”
?[164]
While the jurisprudence describes the approach to anticipation using various expressions relevant to the facts of the cases then under consideration, ultimately it is important to bear in mind that the actual requirement to be satisfied is simply that provided in section 28.2 of the Patent Act. At its simplest, the assessment of anticipation can be reduced to this: the subject-matter of the claim being examined is analysed in order to identify the elements that are essential to the applicant’s proposed solution to the problem being addressed by the application. The prior art is analysed to determine if it discloses and enables the use of the same elements (whether or not disclosed in the same terms) in a form suitable for the same purpose as the claimed matter. If so, the prior disclosure anticipates the later claimed subject-matter.
In performing this analysis, it may be necessary to determine whether claimed elements function in combination to produce a unitary or synergistic result. Where different elements or sets of elements in a claim operate independently of each other to produce distinct results, then the two do not form a proper combination, but rather define an aggregation. In such a case, the removal of one element would have no effect on how the remaining elements function. Where a claim is construed to define two or more collocated but distinct inventions, each invention should be individually assessed for anticipation. In such cases, a defect under section 28.2 of the Patent Act should not be raised unless all of the inventions are anticipated; if at least one invention is novel, the claimed subject-matter will not have been previously disclosed.
In assessing anticipation, it may also be determined that a claim encompasses many different operating embodiments. The claim will be anticipated if any one working embodiment is disclosed and enabled by the prior art.[165]
Example:
An application is directed to improved methods of preparing rigid polyurethane foams with good insulating values. The application discloses that the inventors set out to improve the insulating values of rigid polyurethane foams by preparing them in the presence of a blowing agent comprising a perfluorocycloalkane and a straight-chain alkane in specific ratios. The application teaches that water may be used as a co-blowing agent.
Canadian application D1, filed by a third party before the claim date of the application but published later, discloses the use of a blowing agent falling within the ranges disclosed and claimed in the application in the preparation of rigid polyurethane foams. D1 is silent as to whether water should be used as a co-blowing agent. Because of its filing and publication dates, D1 is relevant for first-to-file anticipation under paragraph 28.2(1)(c) of the Patent Act but may not be considered when assessing obviousness.
Claim 1:
A method for producing a rigid polyurethane foam, comprising the step of contacting a polyol and an isocyanate in the presence of a blowing agent, wherein the blowing agent comprises a perfluorocycloalkane and a straight-chain alkane in a ratio of x:y and wherein the blowing agent comprises 0.05 to 0.95 wt.% water as a co-blowing agent.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is a chemist knowledgeable in the field of rigid polyurethane foams, including their properties and how to prepare them.
Common General Knowledge (CGK)
It is common general knowledge that foams with good insulating properties can be prepared in the presence or absence of water as a co-blowing agent. Prior art documents D2 to D5 are representative of the CGK of the POSITA, all relate to rigid polyurethane foams prepared by related blowing agents, and further disclose that foams with good insulating properties can be prepared in the presence or absence of water as a co-blowing agent. Furthermore, these documents note that water is usually present in small quantities due to the hydrophilic nature of the polyol component used to prepare the foams.
The Problem
It is clear from the description that the problem to be solved was how to improve methods of preparing rigid polyurethane foams having good insulation values.
The Solution
The solution as detailed in the description is to prepare the rigid polyurethane foams in the presence of a blowing agent comprising a perfluorocycloalkane and a straight-chain alkane in specific ratios.
What are the essential elements?
In order to solve the problem of preparing rigid polyurethane foams having good insulating values, the following elements of the claim are considered essential:
contacting a polyol and an isocyanate in the presence of a blowing agent comprising perfluorocycloalkane and a straight-chain alkane in specific ratios.
The following element is non-essential to achieving the proposed solution:
the use of 0.05 to 0.95 wt. % water as a co-blowing agent.
Although the claimed method recites the use of water as a co-blowing agent, it is clear that the common general knowledge of the person skilled in the art includes the knowledge that rigid foams with good insulating properties can be prepared in the presence or absence of water as a co-blowing agent. This is consistent with the teachings of the application’s description, which discloses that water “may” (not “must”) be present, and which does not disclose any specific new results arising from the presence of water. The person skilled in the art would understand that the presence of water is not an essential element of claim 1.
Is the claim anticipated?
Yes, based on a comparison of the elements essential to solve the problem the inventors set out to address, the method of claim 1 is anticipated by the enabling disclosure of D1 under the first-to-file provisions of paragraph 28.2(1)(c) of the Patent Act.
Date modified: June 2016
Although the majority of prior art consists of written disclosures, the sale or use of an invention can also be relevant prior art if it effectively provides an enabling disclosure of the application’s claimed subject-matter prior to the claim date of the pending application.[166]
To be considered to have disclosed the claimed invention, the prior sale or use must provide to the person skilled in the art information sufficient to comprehend the invention.[167] “The use of a product makes the invention part of the state of the art only so far as that use makes available the necessary information.”
[168] The information made available must be such that if the person skilled in the art were to write down that information, they would have drafted a clear and unambiguous description of the claimed invention.[169] Disclosure may be made if the public has the “opportunity to access the information that is the invention”.[170]
As was noted in Baker Petrolite Corp. v. Canwell Enviro‑Industries Ltd., in determining whether a publicly available product anticipates a claimed invention, the ability of the person skilled in the art to reverse engineer the product “in accordance with known analytical techniques” may be relevant.[171] What is required for this consideration is the ability to reverse engineer without inventive effort; it is not necessary to establish that the product was actually reverse engineered.[172]
In considering whether anticipation by prior sale or use of an invention has occurred, the grace period provided for in paragraph 28.2(1)(a) of the Patent Act applies in respect of any making available of the invention by the applicant or by a person who obtained the relevant knowledge directly or indirectly from the applicant (see 18.04).
Date modified: October 2019
An enabling disclosure is considered to disclose everything that would inevitably or necessarily occur or be done by a person practising the invention. Old and known subject-matter is not rendered novel simply by disclosing and claiming a feature which is inherently (i.e. necessarily present) or implicitly (i.e. suggested but not directly expressed) found in the prior art.[173] The concepts of inherent and implicit disclosure are related.
Inherent features of a disclosed invention include properties and characteristics of the elements of the invention, such as the ductility of a metal used in a part in a machine, the mechanism of action of a drug taken to treat a disease, or the thermoplastic properties of a polymer.
Implicit features include those things that a person skilled in the art would, in view of their common general knowledge, necessarily understand to be part of what one would do in order to operate the disclosed invention. If a chemical process calls for ‘distillation at reduced pressure’ without further elaboration, the use of some means for reducing the pressure to below atmospheric is implicit. If a watch band is to be assembled using parts that interact to give the band greater flexibility, the use of attachment means to hold the parts together would be understood by the person skilled in the art and could be considered implicit in the disclosure even if no specific directions to attach the parts together were given.[174]
The mere discovery of the properties of a previously disclosed invention does not make that invention newly patentable, but where the discovery leads to a new practical application of the previous invention that new practical application may be patentable.[175]
Example:
Consider that a prior art document discloses a chemical compound X and how to make it, and establishes that compound X is useful in treating disease Y. Where subsequent research uncovers the mechanism of action of the compound, a claim to the use of compound X to treat disease Y via the newly discovered mechanism is not novel. Compound X inherently treated disease Y via the mechanism, and the discovery has not led to a new use for the known compound.[176] However, if the discovery of the mechanism allows one to conclude that compound X would also be useful in treating disease Z, the use of compound X to treat disease Z may be patentable.
Where features implicit or inherent in a previously disclosed invention are being considered when assessing anticipation, it is important to recognise that such features do not create a new invention if a person using the previously disclosed invention would already have achieved the benefits arising from the presence of the implicit or inherent features. This follows from the “well-known principle in Patent law that a man need not state the effect or the advantage of his invention, if he describes his invention so as to produce it”
.[177] The earlier invention is sufficiently disclosed even if all its advantages were not taught, and the earlier inventor “is entitled to its benefit even if he does not fully appreciate or realize the advantages that flow from it or cannot give the scientific reasons for them”
.[178] Performing the earlier invention would provide the benefits arising from the implicit or inherent features; under the principle of anticipation by reverse infringement [18.01.02], the earlier disclosure would be anticipatory.
Where a conclusion of anticipation requires the presence of an inherent or implicit feature, it is necessary for the examiner to clearly explain the basis for concluding that the feature is implicit or inherent to the matter of the prior disclosure. Where such a conclusion is supported by secondary references, the date of publication of these references is not important.
Example:
In the field of respiratory diseases, the use of a powdered drug C is well known.
An applicant files an application A, which describes a powder inhaler capable of aerosolizing and delivering powdered medicament to a recipient. Their specification describes and illustrates the inhaler as having means for varying airflow volume and resistance and notes that adjustments thereto may be made for delivering unspecified powdered medicaments. No indication is made as to the specific airflow properties of the inhaler but feature Z is illustrated.
Two years after the publication of application A, the applicant files application B, which describes a delivery-efficacy testing regimen for the inhaler claimed in application A. Application B does not describe any inventive medicament, but does refer to drug C. No modifications to the inhaler are disclosed in application B.
Claims of application B:
Analysis: Application B discloses that the dry powder inhaler described in application A was used for the applicant’s trials, and does not describe any modifications made to the inhaler. It must be concluded that whenever the dry powder inhaler of application A is used, it will have the delivery efficiency, flow resistance and flow rate defined in the claim. These are merely inherent properties of a dry powder inhaler as described in application A. Inclusion of these properties in the claim of application B does not direct claim 1 to a different dry powder inhaler than the one disclosed in application A. Claim 1 is therefore anticipated.
Claim 2 defines the dry powder inhaler of claim 1 wherein the drug that will be delivered is the well-known drug C. Since no adaptation of the inhaler is, in view of application B, required for it to deliver drug C, the claim remains directed, simply, to the inhaler disclosed in application A and is anticipated. Defining that the inhaler is capable of delivering drug C merely specifies one of its inherent abilities.
Date modified: June 2016
Anticipation assesses whether a single prior disclosure both revealed the invention in a claim being examined and enabled a person skilled in the art to operate it.
In some limited situations, a single prior disclosure can comprise teachings in more than a single document. This may occur where a primary source of information makes explicit reference to specific teachings in a secondary source, thereby making clear to the skilled reader that the teachings of the secondary source are to be relied on in order to understand or complete the disclosure of the invention in the primary source.
In order to consider multiple sources of information to comprise a single disclosure, there must be an unambiguous relationship between the two sources. References in one source that merely mention the other are not sufficient to establish such a relationship. Rather, the first source must direct the reader to use the teachings of the second source for the purposes of understanding and operating the invention.
Date modified: June 2016
The requirement that an invention be inventive was, prior to October 1, 1996, recognised judicially as inherent to the definition of invention[179] but is now more formally reflected in the Patent Act.[180] Ingenuity is tested by determining whether the claimed invention is obvious (i.e. uninventive) when considered by a person skilled in the art in light of their common general knowledge and the state of the art as a whole.[181] In contrast to the approach for assessing anticipation [see 18.01.02], the evaluation of obviousness allows for a consideration of the combined teachings of multiple prior art documents that the person skilled in the art would discover in a “reasonable and diligent search”.[182]
The use of the term “obvious” in section 28.3 of the Patent Act has not changed the inherent requirement that an invention be the result of ingenuity.[183] The courts have noted that “obviousness is an attack on a patent based on its lack of inventiveness”
[184] and “[t]he courts have chosen to define ‘lack of inventiveness’ rather than ‘inventiveness’ and have called it ‘obviousness’ ”
.[185]
Obviousness is assessed on a claim-by-claim basis by asking whether the claimed invention is obvious (or uninventive) when considered by the person skilled in the art in light of their common general knowledge and the state of the art as a whole.
As with the assessment of anticipation, the assessment of obviousness is based on the elements which would be recognised by a person skilled in the art as providing the solution to a given problem. There is nothing inventive in adding elements to a claim that are irrelevant to the invention’s successful operation.
To be considered obvious, the teachings present in the prior art must be sufficient so that, if combined, they would lead to the claimed invention (or to a working embodiment within the claim). Furthermore, it must be obvious (i.e. uninventive) to combine the necessary teachings so as to arrive at the claimed invention.
Date modified: October 2019
Section 28.3 of the Patent Act defines what disclosures may be considered for the purpose of assessing obviousness. Although any public disclosure of information may be considered in principle, for practical reasons the assessment is almost exclusively performed on the basis of written disclosures. In summary, this section provides for a grace period with respect to disclosures by the applicant before the filing date and allows any third party disclosure anywhere in the world made prior to the claim date to be considered.
Date modified: October 2019
Paragraph 28.3(a) of the Patent Act provides that
The subject‑matter defined by a claim in an application for a patent in Canada must be subject‑matter that would not have been obvious on the claim date to a person skilled in the art or science to which it pertains, having regard to
(a) information disclosed before the one-year period immediately preceding the filing date or, if the claim date is before that period, before the claim date by the applicant, or by a person who obtained knowledge, directly or indirectly, from the applicant in such a manner that the information became available to the public in Canada or elsewhere.
This provision defines public disclosures by the applicant, or by a person who obtained their knowledge directly or indirectly from the applicant, made before the grace period as prior art for the assessment of obviousness, but excludes these disclosures as prior art if made in the grace period preceding the filing date.
The grace period is one year before the filing date of the application, unless the claim date is earlier than that period, in which case the grace period is the period between the claim date and the filing date [See 18.04].
Date modified: June 2016
Paragraph 28.3(b) of the Patent Act provides that
The subject‑matter defined by a claim in an application for a patent in Canada must be subject‑matter that would not have been obvious on the claim date to a person skilled in the art or science to which it pertains, having regard to
(b) information disclosed before the claim date by a person not mentioned in paragraph (a) in such a manner that the information became available to the public in Canada or elsewhere.
This provision defines any public disclosure made by a third party (i.e. by a person other than the applicant or a person who obtained their knowledge directly or indirectly from the applicant) as prior art for the assessment of obviousness if it was made before the claim date [see 18.03].
Date modified: October 2019
Obviousness is assessed from the viewpoint of the person skilled in the art, in light of their common general knowledge and the state of the art as it was on the claim date. For a claimed invention to satisfy the requirement of section 28.3 of the Patent Act there must be present that “characteristic or quality” (i.e. that “scintilla of invention necessary to support the patent”[186]) which serves to elevate the matter of the claims from mere workshop improvement to real invention.[187]
Although various tests have been expressed for assessing obviousness, the inquiry is not well served by attempting to rigidly apply any one test in all circumstances.[188] It is important to address the question in an informed way and the Supreme Court has endorsed a four-step analysis for this purpose, wherein the first three steps frame the inquiry and the fourth step is to ask the pertinent question.
The four steps in the analysis were set out by the Court in Apotex Inc. v. Sanofi-Synthelabo Canada Inc. as:[189]
(1) (a) Identify the notional “person skilled in the art”;
(b) Identify the relevant common general knowledge of that person;
(2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
(3) Identify what, if any, difference exists between the matter cited as forming part of the “state of the art” and the inventive concept of the claim or the claim as construed;
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
The Supreme Court’s admonition against attempting to apply any one test in all circumstances refers specifically to the question asked at step 4.
The Sanofi four-step analysis will typically be done intuitively and automatically by an examiner. Where there appears to be a disagreement between the examiner and applicant(s) as to whether or not a claim is obvious, the Sanofi four-step analysis should be set out in a report. This analysis must be set out in a pre-final or Final Action report.
To inform the first step of the Sanofi four-step analysis, guidelines for the identification of the person skilled in the art and of the common general knowledge follow. It should be kept in mind that the person skilled in the art and the common general knowledge of said person are considered in many aspects of examination and the following discussion is useful in this regard.
Date modified: October 2019
The specification is to be read and understood from the point of view of the person skilled in the art (POSITA). More information on the POSITA can be found in section 12.02.02b.
The person skilled in the art is presumed to read prior disclosures in the same manner as the specification of the application itself. That is, with a mind willing to understand[190] and desirous of success.[191] In understanding the significance of the prior art, they may apply teachings from one source to another setting or even combine teachings.[192]
During examination, an examiner must attempt to interpret the application and the prior art using the appropriate knowledge that the person skilled in the art would have possessed at the relevant date.
Date modified: October 2019
“Common general knowledge means knowledge generally known by persons skilled in the relevant art at the relevant time.”
[193] The relevant time for the purposes of evaluating obviousness is the claim date.
More information on the common general knowledge is found in section 12.02.02c.
Date modified: June 2016
The inventive concept of a claim is not necessarily the same as the essential elements gleaned following a purposive construction analysis. Construing the inventive concept for the purpose of the obviousness analysis is a separate exercise from claim construction, meaning that the construction of the claims is not determinative of the inventive concept.[194]
Purposive construction is used to determine the essential elements of a claim i.e. those elements that provide the solution to the problem that the inventor set out to solve. In contrast, the inventive concept comprises the feature or features of the claim that appear to be inventive over the common general knowledge and/or which the applicant appears to consider inventive. It should be remembered that the identification of the inventive concept should be based on a reading of the specification as a whole from the perspective of the person skilled in the art, in light of their CGK. The inventive concept may be determined to be a combination of the same essential elements identified during the purposive construction analysis and will generally include at least some of the essential elements, but it might not include all the essential elements of the claim as construed.
Purposive construction is outlined in section 12.02 of this manual.
Date modified: June 2016
At step 3 of the obviousness analysis, the inventive concept of the claim in step 2 is compared to the state of the art to determine whether, or to what extent, an equivalent or similar solution to the problem being addressed by the applicant was known at the claim date. The state of the art refers to the information available to the person skilled in the art in accordance with section 28.3 of the Patent Act, and generally will be identified by reference to specific prior art documents that would have been discovered in a “reasonable and diligent search”.
Should there be no difference between the inventive concept of the claim and the state of the art, the claim is most likely defective for being anticipated or obvious. For example, a claim may be anticipated where there is no difference between the inventive concept of a claim and only one prior art disclosure that is cited as state of the art, provided that the prior art disclosure is enabling. In cases where the prior art disclosure is not enabling, the claim may not be anticipated but may still be obvious. A claim may also be obvious where more than one state of the art document is required to arrive at the inventive concept.
Where differences exist between the inventive concept of the claim and the state of the art, it must be determined whether these differences would have been obvious to the person skilled in the art as of the claim date.
Date modified: June 2016
Once any differences between the state of the art and the inventive concept of the claim or the claim as construed have been identified, it must be determined whether the subject‑matter of the claim is obvious or is the result of inventive ingenuity. This must be done without presupposing that the specific problem addressed by the inventors was recognised in the prior art, so as to avoid adopting an improper “hindsight” perspective. Where the existence or nature of a problem was unobvious, the act of identifying the problem may inform the inventive concept.
As noted above, various tests have been articulated in the jurisprudence in order to answer this question, and the Supreme Court has cautioned that no single expression of this test is likely to apply to all circumstances. Although the test question may be framed taking into account the nature of the specific case in question, one must never lose sight that its purpose is to evaluate the statutory requirement of section 28.3 of the Patent Act, and care should be taken to ensure the question is not phrased in such a way that a different standard is applied.
In answering the question at step 4, the factors to be considered include:
It should also be remembered that “the inventive ingenuity necessary to support a valid patent may be found in the underlying idea, or in the practical application of that idea, or in both. It may happen that the idea or conception is a meritorious one, but that once suggested, its application is very simple. Again, it may be that the idea is an obvious one, but that ingenuity is required to put it into practise. Or, again, the idea itself may have merit and the method of carrying it into practise also requires inventive ingenuity”
.[196]
Where the problem to be solved was already recognised in the art, it may be appropriate to inquire only into whether inventive ingenuity was required to conceive of the claimed solution and put it into practice. Where, however, the problem or its underlying cause was not previously recognised or understood, there may be an invention even where the proposed solution to the newly identified problem would have been immediately apparent to the person skilled in the art. Inventive ingenuity, however, does not exist if the alleged problem never existed and was simply an artificial obstacle or “straw man” developed to imply inventiveness in the proposed “solution”.[197]
The assessment of obviousness is approached by considering the prior art as a whole, and the teachings of several documents may be combined in order to show why the claimed subject-matter is not the result of inventive ingenuity. When combining teachings from several documents, the relationship of the documents to each other, and to the person skilled in the art, must be considered. An explanation as to why it was obvious to combine the teachings may be necessary in situations where it is not self-evidently so. This may be given, for example, by establishing why a motivation to combine the teachings in the cited documents exists, whether based on the teachings of the documents themselves, on the common general knowledge or trends in the field of the invention.
Where a document from outside the field of the invention is relied upon in the analysis, the need to explain why it would be obvious to apply the teachings to the field of the invention is greater.
Example of Sanofi four-step analysis:
An application discloses a method of cleaning lead from the interior of a steam still using a high‑pressure stream of water. Suitable operating parameter ranges are disclosed, encompassing those that were actually used by the inventors to successfully clean a still.
The use of high-pressure water to clean surfaces has many applications, and is used in many environments. A search of the prior art reveals documents D1-D3. D1 teaches a method of removing carbon deposits from the interior of a smoke stack by sweeping a high pressure stream of cleaning fluid over the encrusted surface. D2 teaches a wet abrasion process for removing calcium deposits from tiles, and includes illustrations of distributed and focussed spray patterns and of workers sweeping a sprayer at a surface from a distance. D3 teaches a pressure washer for cleaning barnacles off the hull of a vessel, and discloses interchangeable nozzles attachable to a wand where each nozzle produces a specific spray pattern. Each of the documents discloses operating parameters suitable for its specific environment.
Claim 1:
A method of removing lead residue from the interior of a steam still, wherein a stream of fluid from a nozzle is directed to a surface of the steam still at a velocity of between 300 and 1200 ft/s, with the nozzle held from 1-12 inches from the surface at an angle of between 15 and 45 degrees.
Analysis: The problem addressed in the application is cleaning deposits off a hard surface.
In order to determine whether the claimed subject-matter is inventive, the claim is assessed via the four step method [see 18.02.02].
(1)(a) Identify the notional “person skilled in the art”:
The person skilled in this art is taken to be a technician familiar with high-pressure washing and general removal, i.e., cleaning, of deposits from surfaces.
(b) Identify the relevant common general knowledge of that person:
The common general knowledge includes an understanding of typical operating parameters for pressure-washers, suitable cleaning agents and common applications for such washers.
(2) Identify the inventive concept of the claim in question:
In this case the inventive concept includes all of claim 1: a method of removing lead residue from the interior of a steam still, wherein a stream of fluid from a nozzle is directed to a surface of the steam still at a velocity of between 300 and 1200 ft/s, with the nozzle held from 1-12 inches from the surface at an angle of between 15 and 45 degrees.
(3) Identify what, if any, difference exists between the matter cited as forming part of the “state of the art” and the inventive concept of the claim or the claim as construed:
The inventive concept of the claim differs from the state of the art (D1 to D3) in specifying that the surface to be cleaned is the interior of a steam still and in establishing certain specific operating parameters.
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
The person skilled in the art, presented with a steam still surface requiring cleaning would arrive at the operating parameters defined in the claim without inventive ingenuity or undue burden. The use of a pressure-washer in a steam still is directly analogous to its use in the environments disclosed in D1 to D3, and uninventive in view of those disclosures. No unexpected result arises from operating the washer within the parameters defined in the claim. The subject-matter of the claim is therefore obvious.
Date modified: June 2016
Determining whether a claimed invention is obvious at step 4 of the obviousness inquiry may involve asking whether the claimed subject‑matter is obvious because the route to the invention would have been obvious to try. This approach may be especially pertinent in “areas of endeavour where advances are often won by experimentation”[198] but there are no restrictions on its applicability to specific technologies.[199],
When considering an obvious to try analysis, the following non-exhaustive list of factors is relevant:
When assessing obviousness during examination, these factors may be recast as questions to be considered by the examiner:
For the purpose of the obvious to try analysis, it is not necessary that a particular choice from the available solutions be immediately obvious as providing the claimed subject-matter, nor is it necessary that a particular option be best suited to providing the solution. If none of the limited number of predictable and identifiable solutions is related to the solution covered by the claimed subject-matter, the examiner may conclude that the skilled person would not have deemed the subject-matter obvious to try.
The more difficult it is to arrive at the claimed subject-matter from the limited number of likely solutions, the less likely it is that a conclusion of obvious to try is appropriate. Where the person skilled in the art would need to exercise inventive ingenuity in order to solve problems for the purpose of testing the various solutions, for example, it cannot be considered obvious for the person skilled in the art to have tried that route.
In cases where the obvious to try test may be appropriate, the examiner will objectively determine if the exercise of inventive ingenuity or undue effort were necessary to arrive at the claimed solution. The examiner will take into account the nature of the person skilled in the art and the knowledge and the climate in the relevant field or fields at the claim date. The subjective experience of the inventors will not be considered relevant unless it can be established that it reflects what would have been expected of the hypothetical person skilled in the art.
The existence of motivation, in the broadest sense, to solve problems in the field of the invention through scientific inquiry will generally not be sufficient to sustain a conclusion that the claimed invention is obvious to try. It will usually be necessary to show that there was a more specific motivation to work along similar lines as those pursued by the inventor. The person skilled in the art must have been motivated to conduct experiments in the area of the invention, aimed at solving the same or a similar problem to that addressed by the inventor by identifying a solution such as that defined in the claim under consideration.
The attitudes, prejudices and expectations of the person skilled in the art and their awareness of the trends in their field are relevant factors to consider in assessing subject-matter as obvious to try, and are assessed in light of the state of the prior art.
It should be remembered that obvious to try considerations are used to determine whether the subject‑matter of a claim is the result of inventive ingenuity and, by consequence, is unobvious. Factors 1 to 3 might be thought of as asking whether it was obvious to search for a solution to the problem addressed by the inventors (the motivation factor) and whether the route to the claimed subject‑matter was also obvious. If there was no invention in either conceiving of the solution or reducing it to a practical form, the claimed subject‑matter is not the result of an inventive step and is therefore obvious.
Where the questions in factors 1 and 3 can be answered in the affirmative, and the conclusion when considering factor 2 is that the subject-matter of the claim would be arrived at by routine trials that were not prolonged and arduous, it can be concluded that the subject‑matter of the claim is obvious since it would have been obvious to try to identify the claimed matter from among a finite number of likely solutions one of which more or less self‑evidently ought to work.
Little guidance exists as to which areas of endeavour are those in which advances are often won by experimentation, although it has been commented that in such areas “there may be numerous interrelated variables with which to experiment”
.[201] Where there are a finite number of identified, predictable solutions known to the person skilled in the art and a motivation provided in the prior art to find the solution the application addresses, these factors can be indicative that one is in an area of endeavour where advances are often won by experimentation. The “threshold” question of whether obvious to try is applicable is considered to be inherently addressed when the factors of the test itself are considered.
Date modified: June 2016
As stated in section 18.01.02, elements that cooperate to produce a unitary result must be considered in combination when novelty is being assessed. It is not necessary for any of the individual elements of a claim to be new provided the elements are combined to produce a unitary result that is different from the sum of the results of the elements.[202] Such combinations are patentable whereas “a mere aggregation of elements is not”.[203] The subject-matter of a claim is considered to be a mere aggregation if each of the elements performs its own individual function and if any one element is removed the remaining elements would continue to perform their own individual function.[204]
When an invention is merely a juxtaposition of parts or known devices, and each part or device merely functions as would be expected if it were used on its own, the assembly is not a true combination but is a mere aggregation. An aggregation of old parts cannot form the basis of a patentable invention.
An aggregation should be identified as a defect under section 28.3 of the Patent Act as being obvious. Separate prior art documents may be cited to show that each individual part is known in the prior art.
Date modified: June 2016
In many cases, the ingenuity of an invention is related to its utility. This is particularly the case where some unexpected result is achieved through the subject-matter of the claim. This can arise, for example, where a known product or process is modified in some way that makes it novel and leads to the unexpected result. The unexpected result could be, for example, that the product or process becomes useful for some new purpose or provides some additional advantage when used for its intended purpose. Alternatively, the unexpected result could be that despite simplifying the known product or process (for example, by omitting parts or steps) the utility of the original product or process is retained.
Where the invention lies in discovering that a known thing has properties that make it useful for some new purpose, that mere discovery does not confer patentability on the known thing. The new use may be patented, however, if it is novel and unobvious.
Minor variations in existing inventions, such as the changing of size, shape, proportion or quality,[205] where the result is merely the doing of “the same thing in the same way, by substantially the same means, with better results, is not such an invention as will sustain a patent”
.[206] The substitution of a superior material for an inferior material, where the advantages of the substitution were expected, has similarly been found to be obvious.[207]
Even where the use is different, there must be something unexpected or inventive in play to support a patent. “A patent for the mere new use of a known contrivance, without any additional ingenuity in overcoming fresh difficulties is bad and cannot be supported. If the new use involves no ingenuity, but is in manner and purpose analogous to the old use, although not quite the same, there is no invention”
.[208]
Where a combination of parts is being considered, “[a]ll the elements being old, and the functions to be performed being identical, [it can] be patentable only if it performed the old function in some better or cheaper way than did the earlier machines – there must be a new mode of operation resulting from the combination [...]; it is not invention to combine old devices in a new machine or manufacture without producing some new mode of operation...”
.[209] Absent a new unitary result arising from their combination, an assemblage of known parts is merely an uninventive aggregation [see 18.02.04].
The assessment of utility and obviousness may also be somewhat interdependent where the utility of the invention must be based on a sound prediction, particularly where the information necessary to permit a person skilled in the art to soundly predict that a known thing would be useful for some given purpose forms part of the state of the art. Although in certain situations it may be that an invention either lacks sound prediction or is obvious, it must be remembered that the assessment of sound prediction and the assessment of obviousness are distinct tests.[210] The former is based on the applicant’s own description and drawings, scientifically accepted laws or principles and the common general knowledge of the skilled person, while the latter is based on the state of the art.
Date modified: June 2016
Where the subject-matter of a claim is considered to be anticipated by a prior art disclosure, it will often also be considered to be obvious. The existence of an anticipatory disclosure will typically lead to the conclusion at step 3 of the Sanofi obviousness analysis that there is no difference between the inventive concept of the claim and the state of the art [see 18.02.02].
Where the applicant’s amendments or arguments in response to the examiner’s requisition overcome the lack of novelty defect, the claim may nevertheless remain defective for obviousness.
In the interests of keeping examination efficient, examiners having identified that a claim is defective in view of the prior art need not provide separate analyses for anticipation and obviousness defects where a single analysis is applicable to both assessments. It remains permissible for both defects to be identified in a later report, particularly where the applicant’s amendments or arguments have assisted in more clearly identifying any points of disagreement in respect of the applicability of the cited prior art.
When responding to an examiner’s report identifying a lack of novelty, an applicant may be well served to provide comments explaining why the claimed subject-matter should be considered unobvious even if obviousness was not explicitly identified as a defect in the examiner’s report. While no single test is appropriate in all cases where obviousness is a consideration, the Sanofi four-step analysis outlined in 18.02.02 will generally be used by the examiner. An applicant should consider this approach when formulating an argument.
Where an examiner considers that an impasse is developing in respect of the applicability of the prior art, and that the application is approaching rejection in a Final Action, separate analyses for anticipation and obviousness should be provided. This must be done at least one report before the Final Action. More information on the requirements for issuing a Final Action may be found in section 26.04 of this manual.
Date modified: October 2019
In accordance with subsection 28.1(1) of the Patent Act, the claim date is the filing date unless there is a compliant request for priority to a previously filed application (see Chapter 7), where that application (priority document) was filed within 12 months of the pending application by an eligible person in Canada or by an eligible person in an eligible country, and where the claimed subject-matter of the pending application is disclosed in the priority document. Under subsection 28.1(2) of the Patent Act, where those criteria are satisfied, the claim date is the filing date of the priority document.
For situations where the priority document is the subject of a compliant request for restoration of the right of priority (see Chapter 7), the aforementioned claim date requirement that the application be filed within 12 months of the priority document is satisfied, as the filing date of the pending application is deemed, for that requirement, to be within 12 months of the priority document under subsection 28.4(6) of the Patent Act. As such, successful restoration of priority requests may, if those applications satisfy the requirements set forth in subsection 28.1(1) of the Patent Act, result in applications having a claim date that is more than 12 months before the filing date. In principle, each claim in an application may have a different claim date from all other claims, although in practice it is typical for an application to claim priority from one or two priority documents.
Where a public disclosure would be relevant prior art for the assessment of anticipation or obviousness if a claim’s claim date is the application’s filing date, but not relevant if the claim’s claim date is a specific priority date, it will be necessary for the examiner to obtain the relevant priority document and determine whether the application is entitled to the earlier claim date.
The examiner will verify:
Where the request for priority is compliant, and the filing date of the priority document is within (or deemed within) 12 months of the pending application, the priority is valid only to the extent that the priority document discloses the same subject-matter as is claimed in the application. Where the scope of the teachings in the priority document and the application are different, the claim in the application may not benefit from the earlier claim date. Where, for example, the priority document teaches a specific embodiment and the application claims generalised subject-matter covering the specific embodiment, a claim to the generalised subject-matter may not benefit from the priority date if further support for the generalised subject-matter is not found in the priority document, whereas a claim limited in scope to the specific embodiment disclosed in the priority document would.
Date modified: October 2019
An application which claims priority from two or more prior applications may have multiple claim dates. Where an applicant has requested priority from two or more previously regularly filed applications, subsection 28.4(4) of the Patent Act provides that
(4) If two or more applications have been previously regularly filed as described in paragraph 28.1(1)(a), subparagraph 28.2(1)(d)(i) or paragraph 78.3(1)(a) or (2)(a), either in or for the same country or in or for different countries,
(a) paragraph 28.1(1)(b), subparagraph 28.2(1)(d)(iii) or paragraph 78.3(1)(b) or (2)(b), as the case may be, shall be applied using the earliest filing date of the previously regularly filed applications; and
(b) subsection 28.1(2), subparagraph 28.2(1)(d)(ii) or paragraph 78.3(1)(d) or (2)(d), as the case may be, shall be applied using the earliest filing date of the previously regularly filed applications on the basis of which a request for priority is made.
This has the effect of according the earliest possible claim date for subject-matter claimed in the pending application based on the content of the earliest corresponding priority document.
Date modified: October 2019
Any application filed more than one year before the filing date of a Canadian application may not form the basis of priority for the Canadian application. For greater certainty, applications which have been filed more than one year before filing, but satisfy the requirements of subsection 28.4(6) of the Patent Act have a filing date that is “deemed to be within 12 months”. See section 7.06 for further information.
Where a first application has been filed more than twelve months before the filing date of a Canadian application and a second application having the same subject-matter is filed within the 12-month period before the filing date of the Canadian application, priority cannot be based on the second application, except for subject-matter exclusive to the second application. In practice an examiner would not be expected to search for such documents but may come across them during a typical prior art search.
An exception to this bar is found in subsection 28.4(5) of the Patent Act which provides relief where the first application, filed more than one year before the Canadian filing date, has never been open to public inspection and will never publish.
If the first application has never been open to public inspection and is considered withdrawn, abandoned or refused by the granting authority, an inventor may be entitled to full priority rights based upon the subsequently filed second application or, where no previously filed applications remain, the claim date of the pending application will be the date the application is filed in Canada.
Date modified: May 2014
Under some conditions, priority may be based on continuation or continuation-in-part applications before the United States Patent and Trademark Office. A United States continuation application is an application which has the same specification of an earlier application but contains claims directed to either different subject-matter, i.e., a different invention than claimed in the earlier application or claims a different embodiment of the earlier claimed invention. No new matter is disclosed or claimed. A continuation-in-part application discloses and claims additional subject-matter over the earlier application.
If a Canadian application is filed within one year of a continuation-in-part application, this continuation-in-part application may serve as a priority document for any new matter not disclosed in the original U.S. application from which the continuation-in-part application extends.
Where a Canadian application is filed more than twelve months after the filing date of the original U.S. application, but within twelve months after the continuation-in-part, the applicant is not entitled to priority on subject-matter common to the two U.S. applications, except in circumstances as described below. If both the original and the continuation-in-part applications are filed within the 12-month period preceding the filing of the Canadian application, priority may be based on both the original application and on the new matter in the continuation-in-part.
Where priority is necessary to support a claim date in the prosecution of a Canadian application claiming priority from a U.S. continuation-in-part application only, it is necessary to identify the matter derived from the original U.S. application to determine the priority rights of the applicant. Because a U.S. continuation-in-part application does not identify the new matter added to the original U.S. application, the applicant must submit certified copies of both the original and continuation-in-part applications whenever required to do so by the Office.
Example:
An application is filed on March 1, 2009. In the Petition, the applicant requests priority from a US continuation application filed in the United States on March 2, 2008. The US continuation application is a continuation of a prior US application (the “original US application”) filed before the USPTO on February 1, 2008. In the Petition, the applicant provides the application number, country code and filing date of the US continuation application and requests priority from this application.
Analysis:
The Canadian application will not be granted the priority date of the continuation application as the subject matter of the Canadian application was disclosed on February 1, 2008 in the original US application, which is more than twelve months before the date the application was filed in Canada. Note: If the second US application was a continuation-in-part application, the Canadian application would receive the priority from the filing date of the continuation-in-part only for the subject-matter disclosed uniquely therein (see also section 7.05).
Date modified: October 2019
The Patent Act provides for a grace period before the filing date, during which information that became publicly available due to a disclosure by the applicant, or by a person who obtained knowledge, directly or indirectly, from the applicant, is not considered during the assessment of whether the claimed invention is novel and inventive. The grace period is one year before the filing date of the application, unless the claim date is earlier than that period, in which case the grace period is the period between the claim date and the filing date.
The claim date may be earlier than 12 months prior to the filing date of an application in circumstances where right to priority is successfully restored (see 7.06 and 18.03) or in situations involving prescribed or designated days (see 2.03.03a).
As defined in section 2 of the Patent Act, the term applicant “includes an inventor and the legal representatives of an applicant or inventor”
. The term legal representative itself “includes heirs, executors, administrators of the estate, liquidators of the succession, guardians, curators, tutors, transferees and all other persons claiming through applicants for patents and patentees of inventions or through holders of certificates of supplementary protection”
.
In considering whether the grace period applies to a given disclosure of information, the prior disclosure is only protected by the grace period if the person making the disclosure was, or must be deemed to have been, the applicant or a person who obtained knowledge, directly or indirectly, from the applicant at the time the earlier disclosure was made (i.e. at the date of making available to the public, such as the date of publication or of laying open for public inspection).[211]
The grace period covers any prior disclosure, whether an oral disclosure such as a presentation at a conference or a written disclosure such as an article in a trade journal. Since the majority of disclosures of information relevant to patent examination are publically available documents, the applicability of the grace period is typically assessed by considering whether the application being examined and the prior publication share authors (e.g. whether the inventors were the authors of a prior publication) or, where the prior disclosure is a patent document, had the same applicant. It must be remembered that, in respect of domestic patent documents, the grace period applies when considering anticipation or obviousness, but not when assessing double-patenting.
In cases where the applicability of the grace period is in dispute, the applicant may provide such evidence as they consider appropriate to support a relationship between the author of the prior disclosure and the applicant.
Date modified: January 2016
In order for a prior disclosure to be considered prior art, the date at which it became available to the public must generally be known. Where the exact date on which a disclosure was made to the public is not known, the disclosure cannot be cited unless a reliable basis exists for concluding that the information was available to the public before the relevant date (i.e. claim date).
For patent documents (issued patents and applications), this information is usually known. In other cases, it may be less clear. It will usually be possible to determine the publication date of articles published by reputable journals, magazines and similar publications. In many cases, the actual date of publication will be indicated (either of the article in particular or of the issue of the publication in which it is found). In certain cases, only the month and year of publication will be identified. In such cases, it cannot be presumed that the document became available to the public before the last day of the month. Where only a year of publication is available, it cannot be presumed that the publication date was earlier than December 31 of that year.
There may be methods for establishing earlier actual publication dates, including (in the case of documents available on the internet) establishing dates of first publication via third party archiving services.
Date modified: October 2019
Part of the claim date analysis requires the examiner to determine whether the claimed subject-matter was disclosed in the priority document. For applications where the request for priority was made on or after October 30, 2019, the content of the priority document can be verified using the copy submitted or made available under subsection 74(1) of the Patent Rules. In the event that examination commences before a copy of a priority document is submitted or made available, examination should proceed assuming that the claim date is the filing date until the copy is submitted or made available.
Date modified: October 2019
For an examiner to verify the content of a priority document filed in a language other than English or French, a translation into English or French may be necessary. Such a request can be done via a notice under subsection 76(1) of the Patent Rules. Recognising that translating documents may place a significant financial burden on the applicant, requests for translations should be limited to cases where no viable alternative exists. Where only a part of the document is necessary for examination, an examiner should indicate, wherever possible, in respect of which part or parts of the document the requisition for a translation is being made.
Where a foreign language priority document appears relevant to examination, an examiner should attempt to locate a version of that document in an Official language with which they can work. In this regard, examiners should make use of reliable online translation engines, such as that provided by the Japanese Patent Office (JPO), at least at the early stages of examination.
Where an examiner is working from a machine translation of a priority document, this should be clearly stated in the report. An applicant wishing to rebut arguments made on the basis of such a document, however, may be required to provide a translation of the document to support their arguments.
If the examiner has reasonable grounds to believe that the submitted translation is not accurate then a further notice under subsection 76(2) of the Patent Rules can be sent. In response the applicant must submit either a statement by the translator that to the best of their knowledge the translation is accurate or a new translation into English or French along with a statement by the translator that to the best of their knowledge the new translation is accurate. In the event that a statement by the translator that to the best of their knowledge the translation is accurate is provided with the translation submitted in response to the notice under subsection 76(1) of the Patent Rules, the examiner should not send out a notice under 76(2) of the Patent Rules.
Date modified: October 2019
For applications where the request for priority was made before October 30, 2019, in order to verify the content of a priority document not filed in Canada the examiner should first attempt to acquire the document from a reliable source, e.g. WIPO’s PATENTSCOPE database or the International Bureau. Where the priority document is not retrievable by the examiner or where the content of a non-certified copy of the priority document has been relied upon and some question exists as to its accuracy, the applicant may be requested in accordance with subsection 196(1) of the Patent Rules to provide a certified copy of the priority document or to make a copy of the priority document available in a digital library.
Date modified: September 2017
Double-patenting refers to the judicially recognised proscription against an applicant being granted more than one patent for a single invention.[212] The principles governing the doctrine of double-patenting have evolved in the jurisprudence, which now recognises two branches: “same invention” and “obviousness” double-patenting.[213]
Underlying this doctrine is the recognition by the Courts that “a second patent [can] not be justified unless the claims [exhibit] “novelty or ingenuity” over the first patent”
.[214] In essence, once a patent is granted for an invention, “further invention” is required to support another patent.[215]
The assessment of double-patenting, in practical terms, can be understood as a specialised evaluation of anticipation and obviousness wherein the “prior art” consists solely of one other patent by the same applicant (the “existing patent”). The assessment differs from the statutory assessment of anticipation and obviousness in two important ways:
The assessment involves a comparison of the claims rather than the disclosure, as the claims define the monopoly. However, claim comparison is not done on a literal construction of the claims; claims are to be given a purposive construction based on a reading of the specification through the eyes of the skilled person, taking into account their common general knowledge. If the claims of the existing patent, when understood by the person skilled in the art in light of the common general knowledge on the claim date and the teachings of the specification as a whole, anticipate or render obvious the claims of the application being examined, the claims are not patentably distinct from each other. Granting both sets of claims would therefore result in double-patenting. Where it can be concluded that the claims in an application are “not patentably distinct” from the claims in the existing patent, the test under either the “same invention” or “obviousness” branch of the doctrine of double-patenting would have been met.
As mentioned above, double-patenting only arises when considering patents belonging to the same inventor or applicant as the application being examined.
The meaning of “same applicant” for the purpose of double-patenting is based on the definition of applicant from section 2 of the Patent Act, and therefore includes an inventor and the legal representatives of an applicant or inventor. The term legal representative itself includes heirs, executors, administrators of the estate, liquidators of the succession, guardians, curators, tutors, transferees and all other persons claiming through applicants for patents and patentees of inventions or through holders of certificates of supplementary protection.
In many cases the named inventor(s) and the applicant may be the same, but this is not a requirement. Applicants may have many individuals working on different aspects of related projects and may consequently list different inventors on an application. Regardless of the persons listed as inventors, double-patenting restrictions apply to an applicant as though the same inventors were listed.
The Office takes the position that the doctrine of double-patenting applies if the application being examined belonged to the “same applicant” at any time.
Date modified: September 2017
Overlap is a term of convenience describing the situation in which an operating embodiment in a claim of an application being examined is identical to an operating embodiment in a claim in an existing patent. The embodiment in the existing patent, being the same as that in the application being examined, therefore acts as a bar against the latter; granting that embodiment in two patents would result in double-patenting.
An operating embodiment can be either the entirety of the claimed subject-matter, or one of several alternatives within a claim. In the latter case, it is possible that the overlap between the claims involves only a small fraction of the scope of the claim in one or both documents. Nevertheless, having the embodiment in question be granted in two patents would result in double-patenting.
Overlap may occur in situations where the claims in the application and the existing patent otherwise appear to be directed to distinct inventions. Where overlap is identified between claims in an application and an existing patent, the claim being examined is not patentably distinct from the claim in the existing patent insofar as the overlapping subject-matter is concerned. The claim being examined is consequently defective due to double-patenting. Removing the overlap, such as by deleting the duplicated subject-matter from the application would remove the double-patenting defect.
Example:
An applicant files two applications consecutively (or concurrently as the case may be). One application claiming feature A issues to patent before the other application. The remaining application claims feature B. Each document has a dependent claim that defines A+B. Granting the application would result in double-patenting for the embodiment A+B, but if the dependent claim directed to that embodiment is removed from the application, and presuming B is not obvious in view of A, the double-patenting defect would be removed.
Date modified: September 2017
Double-patenting is often described as barring a second patent in view of an ‘earlier patent’, and the “sin of double patenting”[216] is often described in terms of the problem of evergreening[217] a monopoly by extending the rights in time through the filing of subsequent applications differing only in uninventive details.
It has been noted, however, that a further patent can provide additional rights to the patentee beyond an extension of the term of the monopoly, and that the overriding principle is the need for a further patent to exhibit novelty and ingenuity in order to be justified.[218] The Office takes the position that having more than one patent to a single invention is not permitted by the doctrine of double-patenting, whether or not the further patent extends the term of the monopoly right granted in the existing patent. The Office takes the position that an “earlier patent” is simply a patent that has already issued and which claims an invention that is not patentably distinct from that in the claims of the application being examined.
This position considers a further patent to be an inappropriate extension of rights both in the sense that the rights in the existing patent would not be exclusive to the existing patent (as provided by section 42 of the Patent Act) and that those rights would not be limited to the term of the existing patent (as provided by section 44 of the Patent Act).
It is not necessary for the existing patent to have issued from an application having an earlier filing or claim date than the application being examined. There are many reasons for which a later filed application could be issued to patent before an earlier filed application, including many factors controlled by the applicant (the request for examination date, a request for advanced examination, the time taken to respond to reports, etc.).
The Office takes the position that an extension of rights can occur whether or not the rights conferred by an existing patent are still available to the patentee. The expiry of the existing patent does not alter that the issuance of the existing patent bars the grant of a further patent defining an invention not patentably distinct from that in the existing patent. In such cases, the grant of a further patent would restore rights that had expired or been surrendered, thus extending the patent rights.
Date modified: October 2019
Where two applications belonging to the same applicant define inventions that are not patentably distinct from one another, the examiner will inform the applicant that a potential double-patenting issue exists. Preferably, this is done in reports on both applications (where a report is warranted; see below), in order to ensure the applicant is fully aware of the potential problem. This potential defect is not identified in a requisition under section 86 of the Patent Rules, since it is not an actual defect until one of the applications issues to patent. Rather, the applicant is advised of the potential defect. Where an application is otherwise in condition for allowance, it will not be held back solely because of a potential double-patenting issue (i.e., a theoretical future defect does not delay allowance of an application). This applies to applications that are in a condition for allowance when first examined; applicants should consequently exercise care when filing applications with closely-related claims, to ensure that all the claims to a given invention are included in a single application. Once a first application issues, the subsequent application(s) will contain an identifiable defect.
Double-patenting is identified between an application and an issued patent regardless of whether the potential defect was identified between the applications while co-pending. This is so whether the double-patenting existed at the time the existing patent’s application was allowed, or was subsequently introduced to the application being examined by way of amendment. It is up to the applicant to ensure that all the claims to a given invention are included in a single application. Where a patent issues, but claims to certain aspects of the defined invention were omitted during the application stage (whether accidentally or by design), double-patenting will prevent the granting of those claims in a subsequent patent unless they represent “further invention” over the claims in the existing patent.
Date modified: October 2019
The Supreme Court has noted that if “patents are granted on divisional applications directed by the Patent Office, none of them should be deemed invalid, or open to attack, by reason only of the grant of the original patent”
.[219]
Where an examiner has identified a lack of unity of invention in a report on an application, and the applicant files a divisional application in response to that report, the claims in the divisional application are exempt from examination for double-patenting if they are identical to claims identified by the examiner in the parent application as lacking unity and they differ from those retained in the parent application.
Chapter 21 of this manual details the procedures for identifying a lack of unity among the claims of an application. Subsequent to any divisional applications that result from an examiner’s identification of multiple inventions in a parent application, a double-patenting defect will not be identified where the claims in the divisional application correspond to claims identified in the report as belonging to a different invention than that defined in the claims retained in the parent. This is typically the case where the applicant has adhered to the claim groupings identified by the examiner.
Where, however, the claims in the divisional do not correspond to the groupings identified in the report on the parent application, whether at filing or as the result of subsequent amendment, they will be examined for double-patenting. This is typically the case where the applicant either determines that groupings different from those identified by the examiner are appropriate, or where subsequent to division the applicant amends the claims (in either the parent or the divisional application) so as to change the claimed invention (see e.g. sections 3.04 and 21.07.05, 21.09, 21.10 for further information on divisional applications).
Date modified: June 2016
A selection, as the term is used in patent law, rests on the idea that if a disclosure has provided a general description of an invention (e.g. a genus), it may be that certain things falling within the scope of the general teachings can nevertheless be considered to be different inventions (e.g. a species of the genus). These further inventions must be based on the disclosure of substantial advantages not disclosed by the inventors of the broad invention.
The three conditions that must be satisfied for a patentable selection are that:
It is important to note that the advantage (which can include avoiding a substantial disadvantage) must be in comparison to the overall group from which the selection has been made, and be made on the basis of sufficient representative testing and not simply be a comparison to a few isolated members of the overall group.[221]
It should be remembered that in assessing whether an alleged selection is patentable, the patentability of a claim must also be assessed against the usual requirements (novelty, utility, ingenuity, sufficiency of disclosure, etc.)[222]
A newly discovered, substantial advantage is necessary to provide the utility and inventive step to the selection for patentability to be acknowledged.[223] Although there is no special or higher disclosure burden for a selection in comparison with any other type of invention, the advantage must be properly disclosed for there to be an invention[224] and, if unclear, the new utility arising from the advantage must also be disclosed. If there is no way to assess the purported “advantage”, there is no way for the person skilled in the art to appreciate that an invention has been “correctly and fully” described. An inventor “has in truth disclosed no invention whatever if he merely says that the selected group possesses the advantages. Apart altogether from the question of what is called sufficiency, he must disclose an invention; he fails to do this in the case of a selection for special characteristics, if he does not adequately define them.”
[225]
A purported selection whose utility has not been established, by demonstration or sound prediction [see Chapter 19 of this manual], is necessarily not an invention. Establishing that there is, in fact, an advantage requires that some point of reference be disclosed. Mere statements that a certain embodiment of an identified group is “preferred” or possesses an otherwise unspecified advantage, benefit or improved property are not sufficient to adequately disclose the substantial advantage necessary to establish inventive selection.[226]
The ingenuity of the alleged selection involves a consideration of whether “a particular member or group within [the earlier disclosed] class [has] the same or different properties, and, if different, how different?”
[227] Its novelty rests on the fact that the selected aspects of the prior disclosure had not previously been made: per Maughan J. in I.G. Farbenindustrie, “[i]t must be remembered, of course, that the selected compounds have not been made before, or the patent would fail for want of novelty”
.[228]
If an operating embodiment within the selection claim has already been made, the advantages of the invention have already been made available and the claimed invention is anticipated. If something within the selection claim was merely listed in the prior document, however, without disclosing the advantage upon which the selection is based, the requirement for prior disclosure is not met and there is no anticipation.
Where a purported selection is not anticipated, it may nevertheless be found to be obvious. The assessment of the obviousness of a selection may in some cases be directly assessed by a consideration of whether the alleged advantage is truly unexpected, but may also arise (particularly in the chemical arts) in the context of an obvious to try analysis[229] [see 18.02.03].
Example:
An application discloses that it is known to raise sunken ships by pumping a plurality of buoyant bodies through a tube into the ship, and that in the past this had been done by pumping hollow spheres into the ship. The application discloses the use, in particular, of tetrahedral bodies, whose greater packing density increases the effectiveness of the method.
A search of the prior art reveals the use of buoyant bodies to raise sunken ships, but does not indicate the particulars of the shape of said bodies. One piece of prior art appears to illustrate spherical bodies for this purpose.
Claim 1:
A method for raising a sunken ship, the method comprising the steps of: 1) establishing a conduit between a surface pump and the sunken ship,
2) pumping a plurality of generally tetrahedral‑shaped buoyant bodies into the ship via the conduit.
Analysis: The prior art teaches the use of buoyant bodies in general, but it appears that only spherical shapes were specifically used. The application teaches that tetrahedral bodies have a substantially higher packing factor than spherical bodies and achieve better packing efficiencies than those of rectilinear or curvilinear bodies. The result of using tetrahedral bodies enables greater packing into a sunken ship and thus higher maximum buoyancy as well as substantially greater retention of the buoyant bodies in the ship (i.e., loss prevention). Given the disclosure of an advantage specific to the use of tetrahedral bodies, it appears their use could be approached as a potential selection from among the generic means “buoyant bodies”. Since no prior disclosure of the use of tetrahedra exists, novelty can be acknowledged. The obviousness of claim 1 would have to be evaluated to determine whether the selection of tetrahedra in particular from “buoyant bodies” in general leads to an unexpected benefit such that an inventive step could be acknowledged.
Date modified: October 2019
Where an applicant is aware of relevant prior art at the time of filing, or becomes aware of relevant prior art during prosecution, they may choose to amend their claim in order to exclude certain embodiments disclosed in the prior art.
One method for excluding known subject-matter is by a proviso; a statement that provides that the claim does not include some specified matter. The term proviso is used herein to refer to any such exclusionary limitation, regardless of the precise language used to express it (e.g. an attachment means, provided said attachment means is not a rubber-based adhesive; a straight chain alkyl group other than an ethyl or propyl group; a non-field effect transistor).
A proviso based on a prior art disclosure may be introduced to an application in order to establish novelty. To comply with section 38.2 of the Patent Act the proviso should not introduce new matter (e.g. by broadening the claim outside what was reasonably inferable from the original specification).
A proviso may be used to establish novelty, or inventive step over the prior art. When introduced as an amendment, a proviso that excludes a feature that was not necessarily present in the original claim should be very carefully considered, since the newly-identified feature is presumably not required for the proper operation of the claimed subject-matter.
In general, a proviso will therefore render a claim patentable where the broad claim would have been considered novel and inventive if it were not for an isolated earlier disclosure of something within the claim. A broad product claim might, for example, be anticipated by a specific product suitable for the same purpose as that taught by the applicant, but which was disclosed in the earlier document for a different use. Excluding the specific product might render the remaining subject-matter of the claim novel. Depending on the relationship between the two uses, the proviso might be sufficient to also render the amended claim unobvious.
Where a claim is amended to include a number of provisos to establish novelty and inventiveness, a greater level of scrutiny is necessary to ensure that the remaining subject-matter is still a single invention, and that the nature of the invention described in the original application has not been obscured or changed (e.g. by defining the invention solely in terms of what it is not, rather than what it is).
Example:
An application describes the therapeutic effectiveness of a class of compounds which have, in common, structural element A. Prior art application D1 discloses compound X as a useful drug in the therapy of disease Y, X comprises structural element A. Subsequent to the publication of D1, the applicant found that A is an element essential to the effective treatment of disease Y in the class of compounds.
Claims:
Analysis: Claim 1 is anticipated by D1 because compound X has established utility as an effective treatment for Y and comprises structural element A.
Claim 2 is not anticipated by D1 as the proviso removes the applicability of D1 by tying the effective treatment to previously unknown importance of element A in said treatment.
Having been deemed novel in view of the proviso, the inventive concept of claim 2 would require additional analysis to determine inventiveness in view of the common general knowledge at the claim date.
Information regarding unity and provisos can be found in subsection 21.08.07 of this manual.
Date modified: November 2017
Section 2 of the Patent Act requires that an invention be useful. In AstraZeneca Canada Inc. v Apotex Inc., the Supreme Court noted that “[t]he application of the utility requirement in s. 2… is to be interpreted in line with its purpose — to prevent the patenting of fanciful, speculative or inoperable inventions.”
[230] “For the subject-matter to function as an inventive solution to a practical problem, the invention must be capable of an actual relevant use and not be devoid of utility.”
[231] “…[A]n invention must ‘be useful, in the sense that it carries out some useful known objective’ and is not merely a ‘laboratory curiosity whose only possible claim to utility is as a starting material for further research’”
.[232]
In order to determine whether a patent discloses an invention with sufficient utility under s. 2, the Supreme Court rejected the application of the promise doctrine and set out the analysis that should be undertaken by the courts to correctly approach utility: First, identify the subject-matter of the invention as claimed in the patent. Second, ask whether that subject-matter is useful – is it capable of a practical purpose (i.e. an actual result)?[233]
Utility will differ based on the subject-matter of the invention as identified by claims construction. The scope of potentially acceptable uses to meet the s. 2 requirement is limited – not any use will do. The usefulness of a proposed invention must be related to the nature of the subject-matter and cannot be saved by an entirely unrelated use.[234]
The Patent Act does not prescribe the degree or quantum of usefulness required, or that every potential use be realized. A single use related to the nature of the subject-matter is sufficient and the threshold that must be met to establish utility is quite low; “a scintilla of utility will do”.[235]
Except where utility is the essence of the invention (e.g. new uses for old compounds), an applicant need not expressly set out the utility of the invention in the application;[236] however if an invention’s utility is questioned, utility must be shown to have been demonstrated or soundly predicted (see 19.01.03) as of the application’s filing date. This ensures that patents are not granted where the use of the invention is speculative.[237]
To be directed to a useful embodiment, a claim must define the inventive element or combination of elements necessary to enable the proper operation of the invention for its intended purposes.[238] A feature that is required to allow the invention to work, the presence of which is understood by the person skilled in the art as being implicit, need not be explicitly defined.[239]
Date modified: November 2017
To be considered to have utility an invention must be controllable and be reliably reproducible;[240] the desired result must inevitably follow when the invention is put into practice and may not be left up to chance. It is to be noted that the idea that “the desired result must inevitably follow” can refer to an accepted degree of success of a particular repetitive mass production method. For example, if a method is known and well recognized in a particular art as having a particular ratio of success or a certain percentage of rejects, the desired result inevitably follows if the method's ratio of success is inside such generally accepted parameters or if the method produces a percentage of rejects that is within these known parameters.
Inventions which are arrived at by chance and which cannot be reliably reproduced lack utility.[241] An invention that relies upon the judgment or reasoning of an operator is considered to lack reproducibility and thus, lacks utility.[242] Certain mental steps involving the ascertaining and sensing facilities have precise and predictable results, and do not of themselves cause the art or process that relies on them to lack utility. Whenever a person is called upon to perform a subjective judgement, however, the result will invariably be subject to factors such as intuition, creativity, conjecture and approximation, and the result will not be objectively controllable or reproducible. This lack of control and reproducibility is amplified if the subjective judgement calls into play a person’s system of values, beliefs, interests or preferences.
Date modified: November 2017
The utility of an invention must be established as of the filing date of the patent, either by demonstration or sound prediction.[243] Where an examiner reviewing an application has reasonable grounds to believe that the application does not comply with the utility requirement of section 2 of the Patent Act and in response the applicant provides data to demonstrate utility, the data must show that the utility of the invention was demonstrated as of the filing date. “Unless the inventor is in a position to establish utility as of the time the patent is applied for, on the basis of either demonstration or sound prediction, the Commissioner is “by law” required to refuse the patent.”
[244] “Utility and sound prediction are questions of fact and must obviously be supported by evidence.”
[245]
Where the utility of an invention is to be established by demonstration, the demonstration must have occurred as of the filing date but need not have been included in the description.[246] Information establishing the demonstrated utility as of the filing date may be provided after the filing date by the applicant by way of affidavit.
Where an applicant is called upon to establish utility and proposes to demonstrate that their invention had utility as of the filing date, this demonstrated utility should be established by experimentation and testing of all embodiments of the invention or of all members of a genus claimed, for example, and cannot rely on literal assertions that the claimed invention has utility.
When an applicant is not in a position to demonstrate the utility of the invention, a sound prediction must be relied upon to establish utility. Soundly predicted utility pertains to embodiments of the invention that have not been demonstrated to have utility, but for which an appropriate factual basis exists upon which this utility, across the full scope of the claimed invention, can be predicted. That is, the utility need not have been demonstrated at the time of filing the patent, but the scientific rationale underlying the utility must have been established through a sound prediction at the time of filing.
It bears mentioning that the doctrine of sound prediction is of general applicability in every field for which patent protection may be sought and has, for example been applied in the mechanical arts. [247]
Date modified: November 2017
A sound prediction analysis must consider the following three components:
The factual basis generally comprises the facts regarding the invention that are provided by the applicant in the description and drawings, relevant scientific principles, and information pulled from the common general knowledge of the person skilled in the art. The sound line of reasoning can be thought of as the analysis that sets out how the applicant logically bridges the gap between the factual basis and the purported utility of the invention.
The relevant date for determining whether the prediction is “sound” is the filing date of the application.[249]
It is important to keep in mind that a “sound prediction” by its very definition does not imply certainty; however a sound prediction is not to be diluted to a lucky guess or mere speculation.[250] Consequently, in assessing whether or not utility has been established via a sound prediction the emphasis is appropriately placed on the term “sound”, and the question at hand is whether a prediction is “sound” or “speculative”. In Monsanto Co. v. Commissioner of Patents, Pigeon J. adopted the following terms to express this lack of certainty: "[i]f it is possible for the patentee to make a sound prediction and to frame a claim which does not go beyond the limits within which the prediction remains sound, then he is entitled to do so. Of course, in so doing he takes the risk that a defendant may be able to show that his prediction is unsound or that some bodies falling within the words he has used have no utility or [...] that some promise he has made in his specification is false in a material respect"
.[251]
Date modified: November 2017
Evaluating what will be a sufficient factual basis for a sound prediction must be conducted on a case-by-case basis and will depend on such factors as:
A factual basis does not by necessity mean experimental data[252] and though it may be provided by way of examples there is no absolute requirement that this be so. The factual basis could be found in scientifically accepted laws or principles, in data forming part of the state of the art and referred to in the description, or in information that is considered to be common general knowledge of the person skilled in the art.
The term “factual basis” implies support and proof. As mentioned in 19.01.03, “[u]tility and sound prediction are questions of fact and must obviously be supported by evidence”
.[253] Simple, unsubstantiated statements in the description suggesting that the invention will work are not considered to be factual. Similarly, while an applicant can include “prophetic examples” in their application, they have no value in providing a factual basis for a sound prediction. A prophetic example is by definition a statement of what might be, rather than what is, and therefore is not factual.
Date modified: November 2017
The sound line of reasoning connects the factual basis to the purported utility of the invention. The person skilled in the art must be able to understand how the sound line of reasoning links the factual basis to the purported utility of the invention.
Date modified: November 2017
For there to be a proper disclosure of the sound prediction, the description must provide sufficient information such that a skilled person in the art, in light of their CGK, would understand the basis of the sound prediction and be able to predict that the entire scope of the claimed invention would work once reduced to practice.[254]
With respect to what needs to be disclosed to meet the third requirement of the sound prediction analysis, it is the factual basis and the sound line of reasoning that must be disclosed.[255] The extent to which the factual basis and sound line of reasoning must be described in the original description must be evaluated on a case-by-case basis. Elements of either the factual basis or the sound line of reasoning that can be found in scientifically accepted laws or principles, or which would be self-evident to a person of skill in the art in view of the common general knowledge will not, as a general rule, need to be disclosed in the specification. Information that forms part of the state of the art could, depending on the specific circumstances, be properly disclosed merely by referring to the document in which it is set out. Where such documents are referred to they must be properly identified.[256]
Where a sound prediction relies on additional information that is not publicly available, such information must be included in the description[257] at the time of filing. In contrast with evidence that demonstrates utility, an applicant cannot provide evidence after the filing date to properly disclose a sound prediction, even if the evidence was generated before the filing date. Explanations provided during prosecution as to the nature of the sound line of reasoning can only be considered to the extent that they explain why a person skilled in the art would have appreciated the sound line of reasoning on the basis of the description as filed and their common general knowledge.
Since the disclosure is directed to a person skilled in the art, the disclosure must allow that person to make a sound prediction. It is not enough for the description to disclose information that allows for a sound prediction only when interpreted in view of information not available to the public (e.g. proprietary knowledge possessed by the applicants only), or only when interpreted by an expert having a level of knowledge beyond that expected of the person skilled in the art.
Although an applicant is generally not required to provide a theory of how an invention works, if the utility of the invention is predicated on a sound prediction, and the line of reasoning depends on an understanding of the theory as to why the invention works, it may not be possible to properly express the line of reasoning unless this theory is disclosed.
It is important to note that the disclosure requirement within the sound prediction analysis and the sufficiency of disclosure requirement are distinct and separable requirements.[258] The disclosure requirement within sound prediction analysis is tied to the requirement that an invention have utility as set out in section 2 of the Patent Act; it does not pertain to the sufficiency of disclosure requirement set out in subsection 27(3) of the Patent Act. [See section 14.02.01 for a discussion of sufficiency.]
Example 1:
An application describes the use of a solution comprising cells and a biocompatible, cross-linkable hydrogel-forming polymer to produce, when injected into a patient, a tissue-equivalent (which comprises a three-dimensional (3D) open-lattice hydrogel with cells dispersed therein). The description states that any biocompatible, cross-linkable hydrogel-forming polymer is suitable to create the tissue-equivalent. Also disclosed is detailed information concerning the preferred types of biocompatible, cross-linkable hydrogel-forming polymers that can be used and the specific structural features of each of these types of polymers that make them suitable for use. In particular, the description demonstrates that a tissue-equivalent is successfully formed using a calcium alginate polymer and osteoblast cells.
Claims:
A use of a cell-polymeric solution for injecting a cell suspension into an animal under conditions which cross-link the cell-polymeric solution within the animal to form a three-dimensional open-lattice structure having cells dispersed therein, the solution comprising a biocompatible hydrogel-forming biopolymer which can be cross-linked via covalent, ionic, or hydrogen bonds to create a three-dimensional open-lattice hydrogel which entraps water molecules to form a gel, mixed with osteoblast cells.
The use of claim 1 wherein the biocompatible hydrogel-forming biopolymer is calcium alginate.
Analysis: claim 1 encompasses the use of any biocompatible cross-linkable hydrogel-forming biopolymer to create a three-dimensional open-lattice hydrogel for the delivery of osteoblast cells into an animal, while claim 2 is limited to the use of calcium alginate as the biopolymer. Where the utility of an invention is called into question, the applicant must be in a position to show that they had either demonstrated or soundly predicted the utility of the invention as of the filing date. In this case, the examiner questions the utility of the invention and notes that the description demonstrates that calcium alginate is a suitable biopolymer for the creation of the desired open-lattice hydrogel. As such, the utility of claim 2 is considered to be established by demonstration and is compliant with section 2 of the Patent Act. What is not demonstrated with respect to claim 1, however, is the use of other suitable polymers, other than alginate. In view of this, it is apparent that the utility over the full scope of claim 1 has not been demonstrated and must, therefore, be established on the basis of a sound prediction.
In order for the prediction to be “sound”, there must be a factual basis for the prediction, an articulable and sound line of reasoning from which the desired result can be inferred from the factual basis, and there must be a proper disclosure of the factual basis and sound line of reasoning.
The factual basis disclosed in the description includes the fact that alginate, which is a biocompatible polymer that can be cross-linked to form a 3D open-lattice hydrogel, was successfully used to achieve the desired result (i.e., creating a tissue-equivalent). Further, in this case it is part of the common general knowledge of a person skilled in the art that the open-lattice hydrogel-forming ability is not unique to the alginate polymer as it is a property shared by many other known biopolymers that are capable of cross-linking through covalent, ionic, or hydrogen bonds.
The line of reasoning is articulated to be that the presence of specific structural features relating to biocompatibility and cross-linkability are required in a polymer in order to produce a 3D open-lattice hydrogel. Given the fact that alginate has these structural features and forms the tissue-equivalent, the applicant states that other polymers having the same specific structural features would also produce a 3D open-lattice hydrogel and deliver the same result. In view of the factual basis, the line of reasoning is considered to be sound and both the first and second requirements of the sound prediction analysis have been satisfied. Given that elements of both the factual basis and the sound line of reasoning that were not CGK to the POSITA were disclosed in the description at the time of filing, the requirement for proper disclosure is also met. Therefore, claim 1 satisfies the utility requirement of section 2 of the Patent Act.
Example 2:
An application describes a method of controlling weeds in a wheat field wherein wheat plants comprising a mutated acetohydroxyacid synthase (AHAS) gene have increased tolerance to imazapyr, an imidazolinone herbicide. The description subsequently states that any mutation in Domain A of the AHAS gene in wheat will confer imazapyr resistance to the wheat. This mutation in wheat allows for weeds to be selectively targeted when the imazapyr herbicide is applied to a field containing both weeds and the mutant wheat plants. Both the wild-type AHAS gene (SEQ ID NO:1) and a mutant AHAS gene having a single nucleotide substitution in Domain A (SEQ ID NO: 2) are disclosed. The description demonstrates that wheat plants comprising the mutant gene of SEQ ID NO:2 are resistant to the imazapyr herbicide, while the wheat plants comprising the unmodified wild-type AHAS gene (SEQ ID NO:1) show susceptibility to the herbicide. The application makes no mention of any other mutations in Domain A of the AHAS gene, nor does it include any information regarding why the applicant believes that any such mutation would lead to the increased tolerance to the imazapyr herbicide.
Claim:
Analysis: the application claims that a method of controlling weeds in a wheat field using the imazapyr herbicide will be effective where wheat plants in the treated field comprise any mutation in Domain A of the AHAS gene. The description demonstrates that one particular mutation in Domain A of the gene, as depicted in SEQ ID NO:2, successfully provides resistance to wheat plants when exposed to imazapyr. What has not been demonstrated, but which is covered in the claim, is that any and all possible mutations in Domain A of AHAS would result in the utility of imazapyr herbicide resistance. In view of this, it is apparent that the utility has not been demonstrated over the full scope of the claim and since the examiner questions the utility, it must be established on the basis of a sound prediction.
The factual basis disclosed is that the AHAS gene is associated with the imazapyr herbicide and that a specific Domain A mutation, as defined in SEQ ID NO:2, confers increased tolerance to said herbicide. These facts alone, however, are not enough to soundly predict the utility of all possible Domain A mutations. The person skilled in the art, in light of their CGK and the information provided in the description, would recognize the complexity and unpredictability of gene expression, and as such, would not be led to extrapolate that the specific Domain A mutation of SEQ ID NO:2 is a reasonable predictor that all possible mutations in Domain A would confer a similar tolerance. As such, there is no articulable and sound line of reasoning.
Therefore, in the absence of an articulate sound line of reasoning, the skilled person could not soundly predict that any and all mutations in Domain A of the AHAS gene would have the disclosed utility. As such, the claim is defective under section 2 of the Patent Act because the utility has not been established on the basis of either demonstration or sound prediction over the full scope of the claim.
Date modified: January 2009
Where a proviso has been presented to avoid inoperative subject-matter, the basis upon which the utility of the remaining matter of the claim has been established must be reconsidered. Since utility will often be based on a sound prediction, a proviso to exclude a known inoperative embodiment requires that the line of reasoning upon which the utility of the remaining matter of the claim is based be reassessed.
Date modified: November 2017
Where there is evidence of inutility in respect of the subject-matter claimed, or where an examiner questions the purported utility and determines that the applicant has not established that utility, either through demonstration or sound prediction, a defect will be identified under section 2 of the Patent Act. A defect may also be identified when the description fails to demonstrate or soundly predict the utility over the entire scope of a claim.
Where an examiner determines that utility is not established by a sound prediction, the examiner must include supporting arguments which detail how the defect is related to the three step test of sound prediction as set out in section 19.01.03.
It should be noted that evidence of inutility can be provided at any time and there is no requirement that such evidence existed at the time the application was filed.
As mentioned above, care must be taken to ensure that the disclosure requirement of sound prediction is not confused with the sufficiency requirement under subsection 27(3) of the Patent Act. An examiner may determine that there is a separate and independent defect under subsection 27(3) of the Patent Act if the description fails to sufficiently disclose the invention, or if the person skilled in the art could not put it into practice without undue experimentation or without exercising inventive skill. Where both defects are presented in an examiner’s report the report must clearly identify both defects and provide separate supporting arguments for each.
On occasion, an examiner may be presented with an alleged invention that is contrary to known scientific principles. Unless the proper operation of such an invention can be established by demonstration (and the applicant can show that it was, in fact, demonstrated at the time of filing), the claims defining it are identified as defective under section 2 of the Patent Act.
Date modified: October 2022
Under the Patent Act, the specification and drawings of an application may be amended, as long as the amendments, inter alia, do not contain new matter when compared to what was filed originally. Subsections 38.2(2) to 38.2(4) of the Patent Act and sections 155.6 and 155.7 of the Patent Rules provide limits on what matter can form part of an amendment, anything outside of which is considered new matter.
Date modified: October 2022
These requirements apply to all applications originally filed in English or French and that are not divisional applications.
According to subsection 38.2(2) of the Patent Act, the specification and drawings may not be amended to describe matter that cannot reasonably be inferred from the specification and drawings as originally filed.[259]
Matter pertaining to prior art with respect to the application may be added to the specification and the drawings, however the applicant must acknowledge in the specification that any such matter is prior art.
If an examiner determines that an amended specification or amended drawing includes new matter, the defect will be identified in an examiner’s report and the applicant will be requisitioned to remove the new matter.
Note also that an amendment that results in the removal of matter from the specification or drawings may cause the application to not comply with subsection 38.2(2) of the Patent Act. For example, if the originally filed specification described a component as made of a specific material, an amendment to remove the recitation of that specific material may be considered to describe new matter if it could not reasonably be inferred from the original specification and drawings that the component could be made of material other than that originally stated.
Amendments containing new matter will also be laid open on the date the application is laid open to public inspection or on the date the amendment is placed on file, whichever is later. This could affect the applicant’s ability to later successfully obtain a patent in Canada or elsewhere for an invention relying on the new matter.
Date modified: October 2022
There are specific new matter requirements for applications that are originally filed in a language other than English or French. Those requirements differ slightly for regularly-filed Canadian (non-PCT) applications (see section 20.03.01) and PCT national phase applications (see section 20.03.02).
Date modified: October 2022
Amendments to regularly-filed Canadian (non-PCT) applications that are originally filed in a language other than English or French must meet the requirements of subsection 38.2(3) of the Patent Act, which requires amendments to be reasonable to infer from both:
For regularly-filed Canadian (non-PCT) applications filed in a language other than English or French, the translated documents submitted to satisfy subsection 15(2) or 15(3) of the Patent Rules, replace the originally filed specification and drawings (see sections 3.02.04c and 3.02.03). Consequently examination of new matter will proceed on the basis of these translated documents.
Date modified: October 2022
Subsection 159(1) of the Patent Rules indicates that subsection 38.2(3) of the Patent Act does not apply to PCT national phase applications.
Instead, PCT national phase applications originally filed in a language other than English or French must meet the amendment requirements set forth in subsection 155.6(1) of the Patent Rules. In particular, any amendments to these applications, after they enter national phase, must be reasonable to infer from both:
It follows that, for PCT national phase applications filed in a language other than English or French, amendments cannot be based on untranslated (e.g. non-English, non-French) text matter in the specification or drawings, unless:
There may be circumstances where PCT national phase applications are filed in a language other than English or French, but contain English or French text matter in the sequence listing. Where the sequence listing contains text matter in English or French and that same text matter is provided in another language, for the purposes of assessing new matter under subsection 155.6(1) of the Patent Rules, the other language must be disregarded (subsection 155.6(2) of the Patent Rules).
Date modified: October 2022
If an amendment is reasonable to infer from the originally filed PCT national phase application (which was filed in a language other than English or French), but is not reasonable to infer from the national phase translated text in the specification and drawings due to an error in the translation, there may be opportunity to correct the translation in accordance with subsection 155.2(2) of the Patent Rules.
Where a compliant corrected translation is submitted, it is considered submitted on the date of the original translation. As such, any compliant corrected translation is to be used as a basis for new matter assessment under subsection 155.6(1) of the Patent Rules.
It should be noted that a failure to translate certain terms is not considered an error in the translation, and as such, untranslated terms cannot be corrected within the provisions of subsection 155.2(2) of the Patent Rules.
Date modified: October 2022
The issue of new matter in divisional applications arises upon filing of the divisional application and upon amendment of the divisional application.
Date modified: October 2022
Upon examination, the content of a divisional application is assessed as of its presentation date. This content is compared to the content of the original parent application on its filing date (Where the original parent application is also a divisional application then it is the presentation date of the original parent application that is relevant). Any matter neither contained in nor reasonably inferable from said original parent application is considered to be new.
For regularly-filed Canadian (non-PCT) applications, a divisional application cannot be filed with matter that could not have been hypothetically added to the specification and/or drawings of the original parent application under section 38.2 of the Patent Act. It follows that where the original parent application is also a divisional application then it, in turn, must be assessed for new matter with respect to its original parent application to determine hypothetical compliance with subsection 38.2(3.1) of the Patent Act (see 20.04.02), and this determination must be repeated for every application present in the chain of divisional applications. If the matter is considered new for any application in the chain then it is considered to be new for the divisional application.
Where matter present in a divisional application of a regularly-filed Canadian (non-PCT) application at its presentation date is considered new and it is not admitted in the specification as being prior art, a defect will be identified under section 91 of the Patent Rules.
Date modified: October 2022
For divisional applications of PCT national phase applications, the requirements set forth in section 155.7 of the Patent Rules must be met.
An analysis similar to that conducted for regularly-filed Canadian (non-PCT) applications (see section 20.04.01a) must be conducted to determine if the matter of the specification and drawings of the divisional at the presentation date could be hypothetically added to the parent application (or where the parent application is a divisional, whether the matter could be hypothetically added to its parent application without introducing new matter).[261]
However, since PCT national phase applications have specific new matter requirements allowing for corrections of mis-translations when the original PCT application was filed in a language other than English or French, there is an additional analysis to conduct when those situations arise.
Specifically, there must be a determination as to whether the matter of the specification and drawings in the divisional application, if hypothetically added to the specification and drawings of the original PCT application and the translation of the same would introduce new matter in view of subsection 155.6(1) of the Patent Rules (see section 20.03.02).
Where matter present in a divisional application of a PCT national phase application at its presentation date is considered new and it is not admitted in the specification as being prior art, a defect will be identified under section 155.7 of the Patent Rules.
Date modified: October 2022
Subsection 38.2(3.1) of the Patent Act states that the specification and drawings of a divisional application may not be amended to add matter that (a) could not have been added to the original parent application; and (b) cannot be reasonably inferred from the divisional application at its presentation date. Subsection 38.2(4) of the Patent Act provides an exception for matter that is admitted as prior art. Situations where the original parent application is also a divisional application are more complex and follow an analysis analogous to that explained in section 20.04.01 above.
The requirements of subsections 38.2(3.1) and 38.2(4) of the Patent Act and subsections 155.6(3) and 155.6(4) of the Patent Rules mean matter present in the original parent application but missing from the divisional application cannot be added to the divisional application after its presentation date unless the matter was otherwise inferable from the divisional application at its presentation date, or admitted as prior art.
Date modified: October 2022
Amendments to PCT national phase applications require a similar analysis as is presented in section 20.04.02,[262] however, they must additionally meet the requirements set forth in subsection 155.6(3) of the Patent Rules. Subsection 155.6(3) of the Patent Rules requires a special analysis when the original PCT application was filed in a language other than English or French, in order to allow for the corrections to mis-translations.
Specifically, there must be a determination as to whether the matter of the amendment, if hypothetically added to the specification and drawings of the original PCT application and the text matter of the translation (excluding untranslated matter in the non-language dependent free text of the sequence listing), would introduce new matter in view of subsection 155.6(1) of the Patent Rules (see 20.03.02).
Where new matter is identified in an amendment to a divisional application based on a PCT national phase application, a defect is identified under subsection 155.6(3) of the Patent Rules, or paragraph 38.2(3.1)(b) of the Patent Act.
Date modified: October 2022
For divisional applications filed before October 30, 2019, the matter present in the specification and drawings upon filing of the divisional and the matter introduced to the specification and drawings by an amendment made before October 30, 2019 will be evaluated based upon subsections 38.2(2) and 38.2(3) of the Patent Act in force as of the date of filing of the divisional application or date of amendment, respectively. For greater clarity, any amendments made on or after October 30, 2019 to a divisional application filed before October 30, 2019 will be assessed as detailed in section 20.04.02.
Date modified: November 2013
The Canadian Patent Act and Patent Rules are based in part on the simple premise of one patent for one invention.[263] The concept of unity of invention refers to the requirement that an application claim one invention only. This requirement serves, in part, to ensure that the fees paid by applicants are fairly assessed on a per invention basis.
Requiring that a patent relate to one invention only also provides a measure of clarity to the patent system, by constraining the scope of individual patents. A patent specification directed to a single invention is clearer and more readily understood than one that attempts to describe and define several.
The present chapter deals with the subject of unity of invention from two perspectives. First, the assessment by an examiner of whether or not, for the purposes of examination, an application claims more than one invention, and with the procedures for dealing with an application that does and second, the framework and requirements for the filing of a divisional application to protect an invention other than the invention to which the claims of its parent application are limited.[264] The term “parent” is used to refer to an application that describes more than one invention, and which served as the basis for the filing of a further application (a “divisional” application) to protect an invention other than the one ultimately claimed in the parent.
Note that throughout the chapter the term “invention” is used to refer to subject-matter that an applicant alleges to be an invention (an “alleged invention”). Where, when assessing unity of invention, an examiner identifies a plurality of inventions in a claim set, this should not be taken as a suggestion that all of the several inventions thus identified have been assessed for patentability.
Date modified: November 2013
The basic framework that governs unity of invention is section 36 of the Patent Act, which provides that
(1) A patent shall be granted for one invention only but in an action or other proceeding a patent shall not be deemed to be invalid by reason only that it has been granted for more than one invention.
Unity of invention has been referred to as “essentially a procedural matter”,[265] as it does not of itself give rise to issues of validity. Section 36 of the Patent Act also sets out provisions whereby the claims are to be limited to one invention only and any additional inventions described (or described and claimed, as the case may be) may be protected by the filing of separate and distinct applications therefor. Thus
(2) Where an application (the “original application”) describes more than one invention, the applicant may limit the claims to one invention only, and any other invention disclosed may be made the subject of a divisional application, if the divisional application is filed before the issue of a patent on the original application.
and
(2.1) Where an application (the “original application”) describes and claims more than one invention, the applicant shall, on the direction of the Commissioner, limit the claims to one invention only, and any other invention disclosed may be made the subject of a divisional application, if the divisional application is filed before the issue of a patent on the original application.
As discussed in the following sections, it is important to approach the concept of unity of invention bearing in mind its legal context and purpose, and not to confuse it with the determination of whether or not one invention is “the same” as another such as is done, for example, when assessing novelty or double patenting and during re-issue proceedings.
Date modified: October 2019
In interpreting section 36 of the Patent Act, the term “invention” in the expression “one invention only” is best understood as having a broad meaning. The broad interpretation of the meaning of the term “invention” in section 36 of the Patent Act is reflected in section 88 of the Patent Rules, which provides that
For the purposes of section 36 of the Act one invention includes a group of inventions linked in such a manner that they form a single general inventive concept.
In interpreting the scope of section 36 of the Patent Act, the Courts have ascribed to the term “invention” a meaning different than that provided in section 2 of the Patent Act.[266] The Courts thus spoke of claims to matter in different categories of invention as being “aspects of a single invention”. A similar, broad interpretation of the meaning of “invention” has been ascribed by the Courts in considering other provisions of the Act.[267] It is clear that the Courts have considered that the legislative intent of section 36 of the Patent Act is not fulfilled by interpreting the expression “one invention only” by giving the term “invention” its definition from section 2 of the Patent Act. That is, section 36 of the Patent Act should not be understood to say where an application (the “original application”) describes and claims more than one new and useful art, process, machine, manufacture or composition of matter [...], the applicant shall [...] limit the claims to one invention only [...].
Thus, as directed by section 88 of the Patent Rules, an application will not be considered to claim more than one invention if the subject-matters defined by the claims are so linked as to form a single general inventive concept.
Date modified: November 2013
The 1996 revision of the Patent Act and Patent Rules had as one of its objects the harmonization of the Canadian patent framework with the Patent Cooperation Treaty standards.[268]
This can be readily appreciated by comparing the language of section 88 of the Patent Rules with that of section 13.1 of the Regulations Under the PCT, which states that
The international application shall relate to one invention only or to a group of inventions so linked as to form a single general inventive concept (“requirement of unity of invention”).
The phrase “one invention only” in section 36 of the Patent Act, when understood in its full context and in view of section 88 of the Patent Rules (as discussed in 14.03), has a meaning equivalent to “one invention only or to a group of inventions so linked as to form a single general inventive concept”
in Rule 13.1 of the Regulations Under the PCT.
The result is that the Canadian unity of invention requirement is not “different from or additional to” that provided for in the Patent Cooperation Treaty. Identifying a defect arising from non-compliance with the requirements of section 36 of the Patent Act does not contravene article 27(1) of the PCT.[269]
Additional examples helpful for understanding unity of invention can be found in sections 10.20 to 10.59 of the PCT International Search and Preliminary Examination Guidelines, available on the web site of the World Intellectual Property Organization.[270]
Date modified: November 2013
Assessing whether or not unity of invention exists in a given claim set amounts to determining, having regard to the specification as a whole, whether or not a “single general inventive concept” exists to link the claims.[271]
The inventive concept can be identified by considering the purpose of the invention. The claimed invention should provide a solution to a practical problem, and claims that define that solution or refinements to that solution (or of how it is to be put into operation or manufactured, as the case may be) may all relate to a single inventive concept. Generally, a set of claims will share a general inventive concept if a set of new and unobvious elements is common to each claim in the set, provided the elements in question are those required for the proper operation of the invention in its broadest aspects.
The inventive concept relates to how a result is obtained (i.e. to the inventive aspects of a practical solution to a problem), and not simply to the idea of obtaining the result per se. The correct standard to consider is that of unity of invention (i.e. unity among the solutions to a problem), rather than “unity of result”. Mutually unobvious means (practical forms) for achieving a given result will generally not share a single general inventive concept.
The PCT expresses the concept similarly, in Rule 13.2 of the Regulations Under the PCT, which states that
Where a group of inventions is claimed in one and the same international application, the requirement of unity of invention referred to in Rule 13.1 shall be fulfilled only when there is a technical relationship among those inventions involving one or more of the same or corresponding special technical features. The expression “special technical features” shall mean those technical features that define a contribution which each of the claimed inventions, considered as a whole, makes over the prior art.
The expression “special technical features” used in the PCT Regulations refers to novel and unobvious elements of the claims that are responsible for the proper operation of the invention.
Date modified: November 2013
Claims that have in common a set of new and unobvious elements [as described in 21.05] satisfy the requirement for unity of invention.
The two aspects of the unity of invention requirement can be considered separately as: 1) the need for a common set of elements among the claims, and 2) the requirement that the common set of elements be new and unobvious (i.e. inventive) over the prior art.
The former can be assessed without regard to the state of the art, and is referred to as an a priori evaluation of unity of invention, whereas the latter requires the state of the art to be considered and is referred to as an a posteriori evaluation. A lack of unity of invention is a defect in an application regardless of whether it is identified a priori or a posteriori.
A typical approach for assessing whether the claims have unity of invention is to identify the claim with the fewest elements, and then check to see if those same elements appear in all the other independent claims. The claims may appear to lack unity of invention a priori where no claim defines solely those elements that are common to all the claims, however the absence of such a claim is not determinative since there is no requirement that there be one claim broader than all others, nor that there be only one independent claim in each category of invention [see 21.08.02 for additional guidance on this point].
In assessing whether a common set of elements is present, the language of section 13.2 of the Regulations Under the PCT should be borne in mind – that the claims must include “the same or corresponding special technical features”. The concept of “corresponding” means that two claims can have unity of invention even if they do not share a set of precisely identical elements, but rather share equivalent elements whose roles in the context of the invention correspond.[272]
Any prior art relevant for a determination of anticipation or obviousness under section 28.2 or 28.3 of the Patent Act may be considered in assessing whether unity of invention exists [see chapter 18 of this manual].
Example 1:
[This example sets forth an a priori analysis.]
An application discloses a paint containing a rust-inhibiting substance X, a process for applying said paint with substance X and an electrode arrangement A for applying paint. The electrode arrangement is useful for applying paint in general, and is not required in order to apply the paint comprising substance X (the benefits of having substance X in the paint are unrelated to how the paint is applied).[273]
Claims:
Analysis: An a priori assessment of the claims reveals two alleged inventions: the paint comprising substance X and the apparatus including electrode arrangement A. The special technical feature of claim 1 is substance X. The special technical feature of claim 2 is electrode arrangement A. Substance X and electrode arrangement A do not cooperate in any way. Claim 4 includes the technical features of both claims 1 and 2. Claim 3 makes reference to the technical features of both claims 1 and 2, but it must be determined whether the reference to the paint of claim 1 implies a practical limitation to the structure of the apparatus. If the apparatus of claim 2 is suitable for painting the paint of claim 1 (as it seems to be, in view of claim 4), then claim 3 defines the same apparatus as claim 2 and would lack unity of invention with claim 1 despite the reference to that claim.
There is an a priori lack of unity between claims 1 and 2, since the two claims do not share a technical feature in common. Unity of invention does exist between claims 1 and 4 (on the basis of the paint comprising substance X) and between claims 2, 3 and 4 (on the basis of the electrode arrangement A).
Note that while claim 4 can be included in an application with either claim 1 or claim 2, if it was maintained in the parent and filed in a divisional application the result would be double-patenting. Therefore, the subject-matter of claim 4 may be included in the claims of the parent or of the divisional, but not both.
Example 2:
[This example sets forth an a posteriori analysis.]
The application describes a computer monitor comprising elements A and B, and further discloses that additional elements C and D lead, respectively, to particular advantages.
A search of the prior art reveals document D1, which discloses a computer monitor comprising elements A and B.
Claims:
Analysis: The claims meet the requirement for unity of invention on an a priori assessment, since elements A and B are common to each claim. In view of D1, however, these elements do not provide a general inventive concept that links the claims. To the extent that elements C and D have each been disclosed in the application as leading to particular, mutually unobvious advantages, claims 2 and 3 are directed to distinct inventions that lack unity of invention a posteriori.
If, on the other hand, it is clear to the examiner from the description and/or the prior art that features C and D do not provide inventive solutions to any practical problem facing the art (and are therefore not the result of further invention over the matter of claim 1), such that D1 renders claims 2 and 3 either anticipated or obvious, then only the consequent defects under sections 28.2 and/or 28.3 of the Patent Act should be identified. No defect under section 36 of the Patent Act should then be identified, although the examiner may note the potential lack of unity that might exist once the prior art defects are addressed [see 21.07.03].
Date modified: October 2019
The Office takes the position that the intent of subsection 36(1) of the Patent Act is that where an application describes and claims more than one invention, the claims require amendment so as to define one invention only. A lack of unity of invention among the claims is identified as non‑compliance with subsection 36(1) of the Patent Act and the applicant is notified of the defect and requisitioned to correct it or to submit arguments as to why the claims do comply with section 36 of the Patent Act. This notification is made in an examiner’s report issued under subsection 86(2) of the Patent Rules.
Given that, where a lack of unity of invention has been identified, the examiner cannot be certain which invention the applicant will elect to maintain in the claims, a report identifying non-compliance with section 36 of the Patent Act need only identify this defect. This is an exception to the usual requirement that a requisition under subsection 86(2) of the Patent Rules be based on a comprehensive examination [see section 12.01 of this manual]. In this sense, addressing a question of unity of invention can be viewed as a procedural matter to be resolved separately from the substantive examination of the application.
Where the applicant responds to a requisition identifying a lack of unity of invention by amending the claims in such a manner as to overcome the defect, this determines for that application the one invention only referred to in subsection 36(2) of the Patent Act [see 21.03]. Thereafter, any other invention disclosed may be made the subject of a divisional application. The Office takes the position that, in accordance with subsection 36(2) of the Patent Act, the claims of the original application [see 21.09] under examination may no longer be directed to the matter of any other invention disclosed. In responding to an examiner’s report identifying a lack of unity of invention, the applicant effectively has the right to elect, one time only, the identity of the one invention only that will be the subject of examination in a given application.
Claims resulting from post-election amendments will generally be permissible in the application if they would have had unity of invention with the claims to the one invention only elected by the applicant.
To avoid prolonged debate over unity of invention, where an examiner considers that the claims lack unity of invention and the applicant declines to limit their claims to a single invention, the examiner may refer the application to the Commissioner of Patents for a determination of the issue. Typically, such a referral will not occur until the examiner has advised the applicant of the defect in at least two reports.
This referral will not take the form of a Final Action, since:
Where a review of the application [see 21.07.06] leads to the conclusion that the application complies with section 36 of the Patent Act, the examiner will resume prosecution and consider all the claims on file.
Where the Commissioner reviews the application and has reason to believe that it does not comply with section 36 of the Patent Act, a letter will be sent to the applicant directing that the claims be limited to one invention only. This direction will be made under authority of subsection 36(2.1) of the Patent Act, and is not a requisition under section 86 of the Patent Rules.
Where the applicant’s amendments in response to the letter make the application compliant with section 36 of the Patent Act, examination of the application will continue. If the applicant’s amendments in response to the letter fail to satisfy the Commissioner that the application complies with section 36 of the Patent Act, the application may be refused under section 40 of the Patent Act.
Date modified: October 2022
As noted in 21.07 and 12.03, substantive search and examination of the application may be deferred, and the report may be limited in scope to address only the unity defect. Alternatively, it may be determined that it is more efficient to identify the unity defect in parallel with a comprehensive examination of some or all of the claims.
If examination proceeds on the basis of only some claims (e.g. on the basis of a group identified by the examiner), an indication must be included in the report of the extent of search and examination performed.
The choice of the examiner to search and examine certain claims does not replace the applicant’s right to make their one-time election [see 21.07]. If the applicant elects a different group of claims for prosecution from the one the examiner chose to examine, prosecution proceeds on the basis of the claims elected by the applicant.
Where there are defects in the application that affect the determination of unity of invention, an examiner may refer to these defects in addition to or instead of the lack of unity defect and should set out how the other defects impact the assessment of unity of invention or vice versa. Defects such as lack of clarity in the claims, or prior art that leads to a conclusion of a posteriori lack of unity of invention are illustrative of the types of additional defects whose resolution may impact the determination. To avoid confusion as to the necessary response by the applicant, it may be preferable to identify such defects informally (e.g. in the preamble of the report, or by otherwise explicitly indicating that the defect is not being formally identified), solely to explain the impact they had on assessing unity of invention.
Date modified: November 2013
A report identifying a lack of unity of invention should explain the basis for the conclusion in a manner that will enable the applicant to decide whether and how to limit or divide their claims for further examination. This explanation should identify what the examiner considers the various distinct inventions to be, and should provide sufficient detail so that the applicant can understand why the different inventions do not share a single general inventive concept. Where the defect is identified a posteriori, the prior art supporting this conclusion should be cited in the report and an explanation of the significance of each document should be provided.
Wherever possible, the individual inventions identified should be related to the claims in which they are defined, so that the applicant can group their claims into sets which would be viewed by the Office as sharing a single general inventive concept. This will generally be done in all cases unless attempting to relate each invention to a specific claim or claims would only introduce a lack of clarity into the explanation of the defect. Other than in exceptional cases, the examiner will set out groups of claims that are considered to be directed to one invention only. When creating such groups, the examiner should clearly indicate to which group each independent claim belongs. Unless an explicit indication has been made by the examiner with respect to a given dependent claim, the applicant may presume that a dependent claim belongs to the group in which the claim it refers to is found.
Where a lack of unity exists among the alternatives defined in a single claim, the examiner will, to the extent practical, separate the various inventions into groups. In such a case, unless otherwise indicated by the examiner, a dependent claim belongs to the group in which the alternative it refers to is found.
As a general rule, if the applicant limits the claims in the application to one group of claims identified by the examiner, the application will be considered to have been made compliant with section 36 of the Patent Act. Certain exceptions to this general rule exist, however, such as where a further lack of unity of invention subsequently becomes apparent in view of prior art discovered after the applicant has elected a group of claims for prosecution.
Note that in identifying the various inventions in a claim set, the term “invention” is used as a matter of convenience only, and in no way implies that the subject-matter of any given claim is patentable.
Date modified: November 2013
In general, a lack of unity of invention should be identified in the first report written in respect of the claims that lack unity of invention.
In some cases, an examiner may identify defects in an application that bear on the question of whether the claims have unity of invention (e.g. obviousness, ambiguity, lack of utility or of support). Where the applicant’s response in respect of the other defects is germane to its evaluation, it is permissible for the lack of unity of invention defect to be formally identified in a later report. Whenever possible, the applicant should be advised that the other defects bear on the question of unity of invention.
Since unity of invention is assessed in view of the claims of the application, a lack of unity of invention may be introduced when amendments are made to the claims. Where a lack of unity of invention is introduced by the applicant with an amendment, an examiner may identify the resultant defect regardless of the length of prior examination of the application.
Where prior art raises the possibility of a posteriori lack of unity, but some of the claims in the application are considered by the examiner to be anticipated or obvious in view of the cited prior art, it may be preferable to not identify the lack of unity of invention as a formal defect until the prior art defect has been addressed by the applicant. The applicant’s response to the prior art defect may advance the examiner’s understanding regarding unity of invention. The examiner may draw the applicant’s attention, informally [see 21.07.01] and depending on the circumstances, to the potential unity defect.
If the applicant responds to a prior art objection by amending the claims, and the claims as amended appear to avoid the cited prior art but to lack unity of invention, an examiner may identify the lack of unity defect.
Date modified: October 2019
As with any requisition sent under subsection 86(2) of the Patent Rules, an applicant may respond to the identification of a lack of unity of invention by amending the application (in order to comply with subsection 36(1) of the Patent Act) or by submitting arguments as to why the application already does comply.
Where the applicant amends the claims by limiting them to claims falling within a single group identified by the examiner, the lack of unity defect identified in the issued report will be considered to have been overcome in respect of those claims [see 21.07.02].
Should the applicant agree that there is a lack of unity of invention among the claims, but disagree as to the grouping of claims set out by the examiner, they may respond to the requisition by identifying groups of claims different from those identified by the examiner and electing one of those groups of claims.
Where the applicant’s response to the requisition does not serve to make the claims compliant with the requirement for unity of invention, a further report identifying the lack of unity defect may be sent.
Date modified: November 2013
The applicant will be considered to have elected an invention whenever, subsequent to a report in which a lack of unity of invention was identified as a defect, the applicant limits the claims to fewer inventions than were defined in the claim set with respect to which the lack of unity of invention was identified. It is not necessary for the applicant to explicitly state that they have “elected the invention of Group A” when making an election (although this may certainly be done by the applicant, in the interest of greater clarity).
Where the applicant’s initial election limits the claims to a single invention, this defines the one invention only referred to in subsection 36(2) of the Patent Act [see 21.07].
Where the applicant initially elects more than one group of claims identified by the examiner, or claims belonging to more than one group of claims identified by the examiner, or even submits new claims entirely, any further election that may be necessary (i.e. should the initially elected claims still lack unity of invention) must be made from among the inventions defined in the initially elected claim set.
Date modified: November 2013
As noted in 21.07, where an examiner considers that the claims lack unity of invention and has notified the applicant of this conclusion, but the applicant declines to limit their claims to a single invention, the application may be forwarded to the Commissioner of Patents for a determination of the issue.
Resolving questions of unity of invention should be conducted efficiently, since the substantive examination of the application is delayed by this procedure. Consequently, if an applicant has been notified of a lack of unity of invention defect in at least two reports they should expect that a referral to the Commissioner could be made without further notification.
To ensure consistency and fairness, where an examiner considers that an application should be referred to the Commissioner, they must first submit the application for review by a Unity Review Board (URB). This board will review the application in order to ensure the lack of unity defect was correctly identified and clearly articulated to the applicant, so that the applicant was in a position to successfully respond to the examiner’s requisition.
Where the URB considers that unity of invention exists, the examiner will proceed with the substantive examination of all claims on file.
Where the URB considers that a lack of unity of invention exists, but that further clarification of the matter is necessary (e.g., further reasons for concluding a defect exists, or additional information regarding the identity of acceptable claim groups), the examiner will issue a further report taking into account the observations of the URB.
Where the URB considers that a lack of unity of invention exists, and has been clearly communicated to the applicant in an examiner’s report such that the applicant could have responded successfully to the examiner’s requisition, the application will be forwarded to the Commissioner of Patents for consideration.
Where the Commissioner considers it appropriate, the applicant will be directed to limit the claims under authority of subsection 36(2.1) of the Patent Act. A Notice of Direction will then be sent to the applicant by the Commissioner.
Where the applicant’s response to the Notice of Direction does not satisfy the examiner that the application complies with section 36 of the Patent Act, the application will be forwarded to the Patent Appeal Board for a final review. At this stage, the process resembles the review of a Final Action [see chapter 26 of this manual], given that the Patent Appeal Board may recommend that the Commissioner refuse the application under section 40 of the Patent Act. In accordance with subsection 86(13) of the Patent Rules, an application will not be refused without the applicant being given an opportunity to be heard.
Date modified: November 2013
The following sections provide more specific guidance on assessing unity of invention.
Date modified: October 2019
In general, it can be presumed when assessing unity of invention a priori that claims in the following categories of invention will satisfy the requirements of section 88 of the Patent Rules when present in a single application:
Where the “process for making a product” of (a) or (c) is a “process carried out on an apparatus” within the meaning of (d), claims to the apparatus can be included in a single application with claims to the product, process for making the product and use of the product so long as the product is inventive by reason of properties that arise by virtue of its being prepared using the apparatus.
Note that it is not required that the scope of the claims to subject-matter in different categories of invention be of similar breadth in order to satisfy the requirement of unity of invention. Where the scopes are equivalent, unity will generally exist a priori. Where the scopes are different, unity may still exist.
For example, a broad process for using products could have unity of invention with a narrow product claim defining only a limited number of the products used in that process (see Example 2, below).
Example 1:
An application discloses a fuel burner wherein the use of inlets arranged tangentially to the mixing chamber results in better mixing and more efficient combustion.[274]
Claims:
Analysis: Unity of invention exists, a priori, among claims 1, 2, 4, and 6. The special technical feature apparently common to these claims is the tangential fuel inlets. Claims 3 and 5 lack this feature, or a corresponding feature [see 21.06], and therefore lack unity of invention both with respect to each other and to the remaining claims. A lack of unity of invention might be identified a posteriori once a search of the prior art had been performed.
Example 2:
An application discloses the discovery that certain compounds, some novel and others known, are useful as plant growth regulants. The compounds are disclosed as a genus (a family of molecules) of common formula A, which comprises specific molecules a1, a2, a3, ..., an. Compounds belonging to the sub-genus A’ are disclosed as being novel, and a1 is taught as a particularly preferred embodiment. No prior art is cited against the novelty of the compositions of claim 1.
Claims:
Analysis: The claims all define compounds that share a common structure that is responsible for their plant-growth regulant properties. The discovery that this structure results in plant-growth regulant properties (i.e. the allegedly new use of compounds A) appears to be the single general inventive concept linking the claims. There is a priori unity of invention among claims 1 to 4.
Date modified: November 2013
Since unity of invention is initially assessed a priori in view of the claims and before the prior art is considered, a lack of unity of invention may be identified in a report where the subject-matter of the claims does not appear to share a single general inventive concept.
As noted in 21.05, a single general inventive concept is identified by finding common elements among the various claims. This is generally done by identifying the claim with the fewest elements, and then checking to see if those same elements appear in all the other independent claims. The claims may appear to lack unity of invention a priori where no claim defines solely those elements that are common to all the claims.
An applicant is not required to claim the entire scope of their invention, however, so a claim defining only the common elements is not required in order to provide a linking inventive concept. In performing an a priori assessment of unity of invention, an examiner must consider the teachings of the description and the common general knowledge in the art before concluding that the claims clearly lack a single general inventive concept. If it is clear that the description discloses a particular set of elements that are common to all the claims as being the general inventive concept, unity of invention a priori should be acknowledged.
Where an examiner identifies a lack of unity of invention a priori, an applicant may respond to a report identifying this defect by identifying those features which they consider to be the inventive elements common to all their claims. The examiner may subsequently verify this assertion by performing a search on the basis of those elements.
Example 1:
The application as filed discloses a class of compounds of formula X wherein all members of X are aliphatic organothiophosphates, methods for preparing compounds of formula X and uses of compounds of formula X as insecticides. The description does not suggest that the class of compounds forms part of the invention.
Claims:
Analysis: An a priori assessment of unity of invention presumes the features defined in the claims are those necessary to render the claims novel and inventive. Independent claims 1 and 2 have compounds of formula X in common, but since such compounds have not been claimed it will be presumed (in view of the description) that they are not an invention in and of themselves. The claims therefore appear to lack unity of invention on an a priori basis. Note that no presumption exists that claims to a “method of preparing X” and to a “use of X” share unity of invention [see 21.08.01 for the combinations of claims for which a presumption of unity of invention exists].
If the applicant considers that the class of compounds of formula X are, in fact, novel and inventive, they could respond to a report identifying the apparent lack of unity of invention by asserting that fact. A search of the prior art on the compounds of formula X would validate this assertion. If such a search failed to disclose any relevant prior art, no further searching in respect of the claims would be necessary. If the search identified relevant prior art, the claims would lack unity of invention a posteriori.
Example 2:
The application as filed discloses that a class of known compounds of formula X, wherein all members of X are 3,4-substituted indoles, are 5HT receptor antagonists and are useful as migraine therapeutics and anti‑depressants. The usefulness of 5HT receptor antagonists in treating both migraine and depression is known in the art, but the 5HT-antagonist activity of compounds of formula X had not previously been identified.
Claims:
Analysis: The general inventive concept resident in both claims is the discovery that the compounds of formula X are 5HT receptor antagonists. Although this feature is not explicitly defined in each claim, it is understood in view of the description to be the basis of the invention. When read in light of the description, the claims have unity of invention a priori.
Date modified: November 2013
An invention is something that is, inter alia, new, inventive and useful. The utility of claimed subject-matter can be indicative of whether one is dealing with a single invention or multiple inventions.
An applicant must establish the utility of their invention by either demonstration or sound prediction [see section 19.01.02 of this manual]. In cases where utility is being established by sound prediction, the nature of the prediction can inform the unity of invention inquiry. Where the claims include many embodiments, and the utility of all of these could be soundly predicted using a single line of reasoning founded on a single set of facts, it is likely that unity of invention exists among the claims. In contrast, if different parts of the claimed matter would require significantly different sound predictions to support their utility, it is likely that the claims include multiple inventions and that there is a lack of unity of invention.[275]
Where different embodiments within a given category of invention are claimed (e.g. species within an inventive genus), and the embodiments all share a generic utility, they may be viewed as aspects of a single invention. Where one embodiment has a significantly different utility than the others, it may also be viewed as a different invention.
Consider a drug of generic formula X for treating asthma and a species A within the genus, where A has significantly different utility from a typical drug X. If the substantially different utility exists in addition to the generic utility, the embodiment can be viewed both as an aspect of a single, larger invention and as a separate invention. Such a circumstance arises, for example, in the case of inventions with different levels of preferred embodiments and unity of invention would typically exist in such a case. Consider that species A treats asthma, but without a side-effect common to drug X in general. Species A is an inventive selection from drug X, and could either be claimed in a separate application or in the same application as the genus X.
If the substantially different utility exists in place of the generic utility, however, the one embodiment does not have the same utility as the other embodiments and is, by consequence, a different invention. Unity of invention would typically not exist in such a case. Here, species A turns out to be a very good decongestant but is not useful in treating asthma. It does not share unity of invention with the genus X.[276]
Date modified: November 2013
A Markush group must define a list of alternatives that, for the purposes of the claimed invention, can be viewed as technical equivalents that perform the same function in substantially the same way. The person skilled in the art should expect that one member of a Markush group is directly substitutable for another in operable embodiments of the invention. A Markush group is identified by the form “an [alternative] selected from the group consisting of [a1, a2, a3, an-1], and [an]”.
Markush groups are most common in the chemical arts; a group of chemical compounds may be appropriately defined in a Markush group if each alternative has a common property or activity and either
Where the alternatives defined in a Markush group do not satisfy the requirements of (b), and where unity of invention cannot be established by elements in the claim other than the Markush group, either the shared structure referred to in (a) or its utility in the context of the invention would need to be novel and inventive over the prior art in order to provide unity of invention to the claimed alternatives.
Where a list of alternatives satisfies the requirements set out above, unity of invention will generally be acknowledged whether the alternatives are claimed in the form of a Markush group or not.[277]
Date modified: November 2013
An intermediate that is physically or chemically transformed to produce a final product may be considered to have unity of invention with the final product, despite that the inventive step and utility that support the patentability of the intermediate and final product may be quite distinct from each other.
The intermediate must, necessarily, be useful for producing the final product. It may also have the same utility as the final product, although this is not required.
To have unity of invention with the final product, the intermediate should share with the final product the principal structural elements of the final product or should serve to introduce to the final product a structural element that is essential to its utility. Different intermediates that introduce different structural parts to the final product, however, will generally not be considered to share unity of invention amongst each other.[278]
Furthermore, the intermediate must be a direct precursor to the final product, in the sense of being removed from the final product by only one or a few steps, and must not be a precursor to a subsequent intermediate that is known in the art and that must be produced on the way to the final product.[279]
The concept of “intermediates and final products” is common in chemical synthesis, but could apply in other arts as well.
Chemical examples of intermediates and final products that could be considered to have unity of invention include:
Example 1:
An application discloses an industrially useful triazole compound defined by formula I, and a method for its preparation by ring-closure of a compound of formula II. The critical structure in the triazole product is the combination of the triazole ring (sub-structure A) with proximal substituted aromatic rings (structures B and D). The necessary stereochemistry of the groups A, B and D is provided by a central ring structure C. The description teaches that the ring structure C can be formed by a ring-closing reaction of functional groups E and F, which are present in the immediate precursor to the final product. The only disclosed utility of the intermediate is in the production of the final product.
Claims:
Analysis: Although the core structures of compound I (final product) and compound II (intermediate) differ considerably, compound II is an open-ring precursor to compound I. Both compounds share principal structural elements, namely the triazole A and the substituted aromatic rings B and D. The intermediate structure E-F is, from a chemical perspective, a known precursor for rings of type C. The two structures are, overall, technically closely interrelated and unity of invention exists.[280]
Example 2:
An application discloses two structurally related molecules A and B. Molecule A is a compound with analgesic properties. Molecule B results from selective methylation and acylation of two hydroxy groups on A. Compound B is not an effective analgesic, but has significant bioactivity as a sedative.
Claims:
Analysis: Compound A is an intermediate that is structurally similar to compound B. Claims 1 and 2 share unity of invention, and share unity of invention with claim 3.
Claim 5 defines the use of compound B, and shares unity of invention with claims 2 and 3 (a product, process to produce the product and use of the product – see section 21.08.01). Although claim 5 does not clearly share unity of invention with claim 1, claims 1, 2, 3 and 5 would typically be considered to have unity of invention in a single application (intermediate to produce B, compound B, process to produce B, and use of B).
Claim 4 lacks unity of invention with claims 2, 3 and 5 as it defines a use of intermediate A other than its use in preparing the final product or an equivalent use to the product’s. Claim 4 (use of A) does share unity of invention with claim 1 (intermediate A). If desired, claim 3 could be included in an application with claims 1 and 4 (considering claim 3 to be a use of A), although in practice it would usually be preferable to include claim 3 in the same application as claims 2 and 5 (considering claim 3 to be a process to produce B).[281] Claim 4 could be claimed in a divisional application.
Date modified: November 2013
Some preparative methods will include more than one step that could be patentable independently of the multi-step preparative method as a whole. This applies particularly to multi-step synthetic methods, although in principle the concepts could apply to any multi-step preparative method (e.g. a method of manufacturing).
For the purposes of unity of invention, an application can include a claim to a single inventive transformative step in a method and to any larger method involving that step up to the entire multi-step method. The utility of the transformative step arises from it transforming a precursor (which will be a starting material or intermediate in the overall method) into a product (which may be a further intermediate in the method or its final product). The transformative step will also typically share unity of invention with its product, and may share unity of invention with certain of the product’s precursors (see 21.08.05).
Other individual steps in the method (or combinations of steps that do not include the inventive transformative step), however, will not have unity of invention with the inventive transformative step. The other step or combinations of steps do not share the general inventive concept of transforming the inventive transformative step’s precursor into its product. Products other than those meeting the “intermediate and final product” requirements set out in 21.08.05 will likewise be considered not to share unity of invention with the inventive transformative step and its product.
Consider a multi-step synthesis involving the following steps:
step A transforming 1 into 2;
step B transforming 2 into 3;
step C transforming 3 into 4;
step D transforming 4 into 5; and
step E transforming 5 into 6.
The applicant considers steps A and D to be inventive, as well as the 5-step method as a whole. Starting material 1 and intermediates 3 and 4 are known, while intermediates 2 and 5 and final product 6 are novel.
The application includes claims to step D, to step E, and to intermediate 5 and the closely structurally-related final product 6. Unity of invention can be acknowledged among these claims as involving inventive product 5, a method for producing product 5 (step D), a method of using product 5 (step E) and by virtue of the “intermediate / final product” relationship between products 5 and 6 [see 21.08.05]. Unity of invention could not be acknowledged between intermediate 5 and intermediate 2 because of the intervening known intermediates 3 and 4 [see 21.08.05], nor could individual steps A, B or C be claimed either alone or in any combination other than one ending with step D (i.e. so that the combination could be viewed as a method for producing 5).
It is worth noting that other groups of claims could be identified which would meet the requirement for unity of invention. For example, a claim to the 5-step method as a whole would have unity with a claim to product 6, to intermediate 5 and to any combination of steps that includes step E on the basis of the general inventive concept being “the preparation of 6 from 5”.
Date modified: November 2013
A proviso is a clause added to a claim in order to remove something that would otherwise be encompassed by the language of the claim.
A proviso may be used, for example, to provide or restore novelty in cases where some part of the claimed subject-matter would otherwise be anticipated.
Whether a proviso causes a lack of unity of invention must be assessed on the facts of a given case. A proviso can be thought of as making the subject-matter of the claim “discontinuous”, and in that sense can remove the generality of what would otherwise be a “general inventive concept”.
In assessing whether a proviso will have the effect of removing unity of invention from the claimed subject-matter, the reason for including the proviso must be considered. Where a proviso is used to avoid prior art, for example, the critical question is whether the prior art has simply disclosed an embodiment falling within a claim or has taught the same inventive concept as the application. In the latter case, unity of invention is most likely absent in view of the proviso whereas in the former this may not be the case.
Example:
An application discloses a genus of compounds (compounds comprising the structure of formula I) useful as antibiotics. The inventors have discovered and disclosed a structure-function relationship based on a certain functional group in the genus. The same applicants had, several years earlier, obtained a patent on a species (species A) falling within the genus. At the time the previous patent was obtained, the applicants knew the species was a useful antibiotic but did not know what structure led to the activity.
Claims:
Analysis: The general inventive concept linking the compounds of formula I is the presence of the functional group responsible for their antibiotic activity, coupled with the discovery of the structure-function relationship. The prior patent had not disclosed the structure-function relationship, and although species A would anticipate the broad genus claim in the absence of the proviso, the proviso does not result in a lack of unity of invention among the remaining members of the genus.
Note that if the earlier patent had identified the structure-function relationship in respect of species A, it would imply a lack of unity of invention a posteriori since the role of the functional group in providing antibiotic activity would have been known.
Date modified: October 2019
In accordance with subsections 36(2) and 36(2.1) of the Patent Act, where an application (the “original application”) describes more than one invention, an applicant may file a divisional application to protect described inventions other than the one invention only to which the original application’s claims were directed or, as the case may be, to which the original application’s claims were limited.
For more information on filing a divisional application including the administrative requirements and deadlines see section 3.04.
Date modified: October 2022
Where a request for examination has been made on a divisional application, examination will be conducted in a manner similar to a regular application; however, there are additional requirements specific to divisional applications which must also be assessed. The content of the specification and drawings of the purported divisional application are compared to that of the parent application to determine if the claims of the divisional application are directed to a different invention than the claims of the parent, and if the divisional application was filed with new matter. See section 20.04.01 for information on new matter when filing a divisional application.
If, at filing or during the course of prosecution, the claims in the purported divisional application are not directed to a different invention than those of the parent application, the later-filed application is not a proper divisional application within the meaning of section 36 of the Patent Act. Note that if the filing of a divisional application was “directed by the Patent Office”, the doctrine of double-patenting does not apply between the divisional and any of its parent or sibling applications.[282]
If the purported divisional application has a presentation date after October 30, 2019 and contains new matter with respect to its respective parent application, it is defective under section 91 or 155.7 of the Patent Rules (see section 20.04.01).
Should an amendment introduce new matter to a divisional application, subsection 38.2(3.1) of the Patent Act or 155.6 of the Patent Rules will apply (please see section 20.04.02 for more information on how the requirements relating to new matter affect amendments to divisional applications).
Date modified: October 2010
The purpose of this chapter is to highlight Office practice as it pertains in particular to computer-implemented inventions.
The term “computer” is used in this chapter to refer to an electronic device comprising a processor, such as a general-purpose central processing unit (CPU), a specific purpose processor or a microcontroller. A computer is capable of receiving data (an input), of performing a sequence of predetermined operations thereupon, and of producing thereby a result in the form of information or signals (an output).
Depending on context, the term “computer” will mean either a processor in particular or can refer more generally to a processor in association with an assemblage of interrelated elements contained within a single case or housing.
The present chapter sets out the Office's practice for determining whether or not an invention is statutory and useful. The former requirement can be framed in terms of asking whether or not the invention is proper "subject-matter" for a patent.
Guidance provided herein in respect of “computers” may apply, where the term has been used to refer to a device comprising a processor, to devices such as network servers, personal digital assistants (PDA), multi-function cell phones, and the like, or even to processor-containing televisions, music or video playback devices and appliances such as bread makers or coffee machines.
In certain contexts, the term “computer” may be used to encompass a device interacting with certain ubiquitous peripherals, such as a keyboard, mouse or display, necessary for interacting with the computer itself. In this sense, the term “computer” may refer to a “general purpose computer” such as a desktop or laptop computer capable of receiving input, such as via a keyboard, and providing output, such as to a display means.
Where references are made to software “stored on” a physical memory, these are intended to simply refer to the fact that the physical memory is storing the software. No distinction is made herein between memory types which are best described as having software “stored in” the memory and those that are best described as having the software “stored on” the memory.
In reading this chapter, it should be borne in mind that its purpose is to clarify, through elaboration, the application of the more generic teachings of other chapters to the particular issues encountered with computer-implemented inventions.
Nothing in this chapter should be interpreted as providing exceptions to any practice of general applicability set out in any other chapter. Throughout this chapter, reference is made to the nature of the contribution in a claimed invention. Additional guidance on the contribution approach used to assess whether a patentable contribution has been made can be found in section 12.02 of this manual.
Date modified: October 2010
As with any invention, in order to be patentable under the Patent Act the claimed subject-matter of a computer-implemented invention must fall within one of the five categories found within the section 2 definition of “invention”, namely art, process, machine, manufacture or composition of matter.
The following sections set out how the five categories of invention apply to computer-implemented inventions in particular, and consequently refine the more general guidance provided in Chapter 17 of this manual.
A computer-implemented invention may be claimed as a method (art, process or method of manufacture), machine (generally, a device that relies on a computer for its operation), or product (an article of manufacture). Certain subject-matter relevant in the computer arts may not be claimed as such, including computer programs [22.08.04], data structures [22.09.02], and computer-generated signals [22.09.05].[283]
A guiding principle in respect of computer-related inventions was provided by the Federal Court of Appeal in Schlumberger, which noted that “the fact that a computer is or should be used to implement a discovery does not change the nature of that discovery”
, and also that the presence of a computer cannot effect the “transforming into patentable subject-matter [of] what would, otherwise, be clearly not patentable”
.[284]
Date modified: October 2010
Computer-implemented inventions falling within the category art are typically claimed as methods.
Many methods involve the use of a computer or an apparatus or device including a computer. A method that, on its own merits, would be considered non-statutory does not become statutory simply by virtue of some part of the method being carried out on or by a computer. The method itself, as a whole, must be a solution to a practical problem and must lie within a field of technology.
Claims to computer-implemented methods for playing games or creating works of art do not define inventions that belong to a field of technology and do not come within the definition of invention in section 2 of the Patent Act [see sections 17.03.09 (Games) and 17.03.07 (Fine arts) of this manual].
A method of controlling a computer’s operations so as to achieve a technological result,[285] in contrast, would come within the definition of invention in section 2 of the Patent Act. In such a method, the electronic processes within the computer are considered to satisfy the requirement that the method include (either explicitly or implicitly) at least one act performed by a physical agent upon a physical object, producing in that object some change of condition.
Date modified: October 2010
As noted in section 17.01.02 of this manual, a process implies the application of a method to a material or materials. To be statutory, a process must apply a statutory method.
When assessing the contribution of a computer-implemented process, it must be borne in mind that the necessary ingenuity can arise from the method, from the material or materials, or from the recognition that applying the method to the material or materials leads to an unexpected useful result.
Date modified: October 2010
A device such as a computer, or an apparatus or system including a computer associated with other devices, is generally viewed as falling within the category machine.
Whether or not a claim to a device defines a patentable invention depends on the presence of a contribution in the claimed matter and the nature of this contribution. For a claim to be patentable it must define at least one statutory element that forms part of the contribution. For a claim to a device to be patentable, the device itself must therefore be a contributed practical form. That is, the device must provide a novel and unobvious technological solution to a technological problem.
Determining whether or not this is the case can be performed by assessing the device itself, but in many cases can also be performed indirectly by reference to the method implemented by the device. W here a statutory method is implemented by a computer, apparatus or system, a device capable of implementing the entire method is necessarily a solution to a practical problem. Presuming the device has been specifically modified to implement the method, such that it is novel and unobvious, it will be a statutory contribution. The patentability of a device is not negated, however, from the mere fact that the device is intended to implement or to be used in a non-statutory method. The question to be addressed in such cases remains whether the device provides a novel and inventive technological solution to a technological problem.
Where a device does provide such a solution, its patentability does not depend on whether it was adapted by providing new hardware or by controlling existing hardware in a particular manner by the addition of software or firmware (software programmed into a read-only memory).
Note that the “technological solution to a technological problem” does not have to be in relation to the operation of the computer as a general purpose device (e.g. it is not necessary that a computer be made more efficient or reliable), but could be simply that the general purpose device has been technologically adapted to act as a special purpose device. Thus, presuming novelty and ingenuity, any of the following provide technological solutions to technological problems and would be viewed as contributed devices: a computer programmed to allow its speakers to simulate “surround sound” (known hardware controlled by new software), a computer adapted to operate using two central processing units (new arrangement of known hardware, controlled by new software), a computer programmed to allocate memory to video processing in a manner that increases the efficiency of the device when running several applications (known hardware controlled by new software), and a computer whose motherboard has an inventive new video card slot with a faster data transfer rate (new hardware).
Where a computer or other device does not provide a solution to a technological problem, the computer or device as a whole is not a contributed practical form of an invention. Where such a device is further defined in terms of discrete non-statutory features, the claim would be objected to on the ground that it does not define a statutory “invention” within the meaning of section 2 of the Patent Act. For example, a computer or other programmable device cannot be patentably distinguished from other computers simply on the basis of stored information; the stored information does not cause the computer to become a new and unobvious solution to a practical problem.
Date modified: October 2010
The category manufacture encompasses both processes for manufacturing and the products made by such processes [see section 17.01.04 of this manual]. As noted in 22.02.03, a device including a CPU is generally viewed as falling within the category machine. The category manufacture is therefore considered to apply to computer-implemented inventions either where a computer is used to control a manufacturing process, or where a non-machine computer product is claimed. The principles discussed in 22.02.02 apply equally to computer-controlled manufacturing processes.
The concept of a non-machine computer product applies to a physical memory storing computer-executable instructions. A computer program per se is not statutory because it is disembodied. A physical medium storing the program, however, may be considered a manufacture. The patentability of such products depends on the nature of the contribution, and is discussed in 22.08.04.
Date modified: October 2010
The category of invention composition of matter relates to chemical compounds, compositions and substances and is not of great significance to computer-implemented inventions. A computer-controlled method or process for manufacturing compositions of matter could be evaluated under the category art or process as the case may be.
Date modified: October 2010
A patentable claim must include a statutory contribution. Where a claim is directed to a computer, it must be determined whether the device itself is part of the contribution – that is, whether the computer itself may be considered novel and inventive.
In evaluating whether the computer has been contributed, it is first necessary to identify the essential elements of the device; i.e., those that, as a set, provide a technological solution to a technological problem. For the computer to be patentable, this set of elements must be novel and inventive.
As noted in 22.02.03, where the machine has been specially adapted to implement the entirety of a patentable (statutory, useful, novel and inventive) method, the machine is considered to be a technological solution and is patentable.
Where a machine implements a non-statutory method, in contrast, inventive ingenuity associated with the method per se does not provide the inventive step necessary to support the patentability of a machine implementing that method. The inventive ingenuity necessary to make the machine patentable must arise in relation to adapting the machine to implement the method.
Date modified: October 2010
A computer can be adapted to solve a problem either by its hardware, software or a combination thereof. Where the adaptation is performed via hardware, this will typically permit a structural comparison of the computer to other computers and will facilitate the assessment of novelty and ingenuity.
More often, however, a computer will be adapted via software. In evaluating whether a computer adapted by software is the result of ingenuity, it is useful to draw a distinction between the design of a computer program and the expression of that program in a specific programming language.
Designing a computer program comprises steps such as developing a method to be implemented by the computer and creating flow charts, design diagrams or pseudocode to describe the method steps to be performed by the computer in order to solve a problem. Furthermore, specific operations and their necessary sequence to enable the computer to implement the method are determined.
Once the design is completed, the computer program is expressed as lines of code. Expressing a computer program in a specific programming language, however, is considered to fall within the common general knowledge of an uninventive skilled programmer and is not considered to require inventive effort. This person skilled in the art is considered to be able to express the program in any number of different programming languages without the exercise of judgement or reasoning, and therefore without the exercise of ingenuity. Consequently, the inventive ingenuity necessary to provide patentability to a computer is never found simply in writing computer code to express a developed program.
Date modified: October 2019
A computer program is not, by itself, statutory subject-matter. However, if the result of running the program on a computer is to provide a novel and inventive technological solution to a technological problem, then the program is viewed as modifying the technological nature of the computer as a whole. The program in such cases is not a discrete element of a claim to the computer.
In considering whether a program will bestow patentability on an otherwise-known computer, the goal is therefore to identify whether it provides a novel and inventive technological solution to a technological problem.
In cases where the computer program expresses a statutory method (i.e. a series of steps which provides a technological solution to a technological problem), the program will be considered to be technological in nature. If the method is also both novel and inventive, then the programmed computer would be patentable. Thus, as noted in 22.02.03, where a computer implements the entirety of a patentable method, the computer is patentable. If the method, while technological, is not novel and inventive then it is not sufficient to render the computer patentable. Note that where the computer only implements part of a patentable method, care must be taken to base the assessment only on those parts of the method which take place on the computer, and not on the basis of the method as a whole.
On the other hand, where the computer program expresses a non-statutory method, the non-statutory method itself is not a patentable contribution, regardless of whether it is novel and inventive. The patentability of the computer claims in such cases will depend on additional elements defining how the computer is adapted to implement the method. These additional elements may or may not be novel and inventive, depending on their nature and complexity and the state of the art in programming at the relevant date. Where inventive effort is needed to enable a computer to implement a method in a novel way, a technological solution to a technological problem has been contributed.
In determining whether the program’s design is inventive or not, the examiner will be guided by the description. Paragraph 56(1)(d) of the Patent Rules states that “a description of the invention must be set out in terms that permit the technical problem and its solution to be understood, even if that problem is not expressly stated.”
Thus, it should be clear from the description what technical (technological) problem is being addressed, and what solution is being proposed by the inventors. Where the examiner is considering whether ingenuity was required in reducing an algorithm to a specific series of operations to be carried out by the computer program, the level of detail included in the description will be informative.
Where the application includes no details regarding how the computer program is to operate, this suggests the applicant considers the manner of implementing their method to be uninventive. It can be appropriately concluded by the examiner that there is no invention in the reduction to practice of the method. This conclusion is not prejudicial to the applicant, since even if the applicant were incorrect in considering the development of the program to be uninventive it would nevertheless follow that the description would not be enabling. Given the lack of disclosure, the programmer would be called upon to exercise inventive effort in determining how the program is to operate.
Where a greater level of detail is provided, the examiner must consider whether the specific implementation is an inventive solution to a technological problem in respect of the operation of the computer, and thereby determine if the computer itself has been contributed.
Date modified: October 2010
The following examples illustrate how the guidance in this chapter can be applied in practice, particularly where the subject-matter of the invention lies outside the field of computers per se.
Example 1:
An application discloses the atomic coordinates of protein X and a crystal structure of said protein. A three-dimensional molecular modelling algorithm is applied to the atomic coordinates to determine the spatial coordinates of the binding pocket of protein X and subsequently, in silico screening is performed to search for compounds that interact with protein X.
Prior art document D1 discloses:
Claims:
Analysis: Claim 1 defines atomic coordinates, which are merely descriptive information relating to the protein. The claim is not, by its form, directed to a statutory invention under section 2 of the Patent Act.
Claim 2 defines this information when stored on a carrier. It is statutory in its form, but does not include a statutory contribution (the storage medium itself being, self-evidently, known).
Claim 3 defines a method whereby a computer generates a 3D model of a molecule, analyses the model to identify a binding pocket, and attempts to find target molecules whose structures are complementary to the binding pocket and which will bind to the binding pocket. Several of the steps involve computer operations that could potentially be technological innovations in the operation of a computer, including generating the 3D model (step a), analysing the model to identify a binding pocket (step b), and performing the shape-matching and energy minimization calculations (steps c and d). Claim 3 is directed, by its form, to a statutory method. In view of D1, however, these operations are already known and therefore do not form part of the contribution. The specific atomic coordinates of protein X do not modify the technological manner by which the computer performs the calculations, and therefore the model of protein X is a discrete element of the claim. The model of protein X is not itself a statutory invention (could not be a statutory contribution). After having set out a contribution analysis, in view of D1, the claim can be found defective under section 2 of the Patent Act on the basis that no contributed statutory subject-matter has been defined and the model of protein X is not a statutory invention.
The analysis of claim 3 would be guided by the description of the application. The level of detail provided in respect of how the computer performs the various modelling, analysis, shape fitting and energy minimization steps would be indicative of whether technological obstacles were overcome by the inventors in respect of these operations. A lack of detail, or for example a reference to the known molecular modelling software of D1, would be a strong indication that there was no innovation in how the computer performed these operations. Note that if specific details were given in respect of how the computer operations were performed, these would need to be claimed in order to distinguish the method from that of D1.
Note that the conclusion with respect to claim 3 is arrived at after having performed a contribution analysis, in view of the substance of the claimed invention. This can be contrasted with the statement made with respect to the claim in example 5 in section 23.02.04 of this manual, which indicates only that, by its form, that claim is directed to a statutory method.
Example 2:
An application discloses a vehicle wheel alignment system comprising a vehicle station used for vehicle testing, a set of optical sensors for measuring vehicle wheel alignment angles, an automated tool for adjusting wheel angles, and a computer station. Aligning vehicle wheels is a process which includes measuring and adjusting a number of wheel angles, such as camber, caster and toe angles, as well as the steering axis inclination. The computer runs software which compares angles measured by the optical sensors with manufacturer-recommended specifications stored in a database and produces an output signal which instructs the automated tool to perform a synchronized adjustment of any wheel angles that are outside predetermined limits. The automated tool is a single unit comprising several modules, with each module being capable of adjusting one of the wheel angles.
The prior art search reveals that the following features are known:
The prior art does not disclose an automated tool for the synchronized adjustment of multiple wheel angles, comprising several modules in a single unit wherein each module adjusts a specific wheel angle.
Claims:
means for receiving inputted data,
means for retrieving manufacturer recommended wheel angle values from an electronic database,
means to calculate differences between the measured values of the vehicle wheel alignment angles and the manufacturer recommended angles, and
means to output a signal based on the calculated values to actuate the automated tool in order to synchronously align the vehicle wheel angles.
Analysis: Claim 1 defines a method involving the application of physical steps to solve a technological problem – how to align the various wheel angles synchronously rather than sequentially. The method, when considered as a whole, is statutory in form. The prior art discloses measuring wheel alignment angles, comparing the measured values to a database and performing the alignment sequentially in respect of each angle. There is no prior disclosure of performing the alignment synchronously. The patentability of the method depends on whether the examiner considers step f, which is novel, to also be inventive. Since the patentability of this claim depends on whether a statutory step is considered to be inventive, the critical assessment can be made under section 28.3 of the Patent Act.
Claim 2 defines a system to perform the method of claim 1. If the system has been specifically adapted in order to perform the method (in this case, the use of multiple modules in a single unit suggests that this is the case), then its patentability depends on the same factor of inventiveness as claim 1. As noted in 22.02.03, a machine specifically adapted to perform the entirety of a patentable method is patentable.
Claim 3 defines a method for performing calculations in order to obtain information. By its form, the claim includes physical steps that could, in theory, be patentable. It is clear, however, that the technological aspects of each step (how to input data on a computer, how to search databases, how to solve a simple algebraic equation on a computer, how to display a result) are known and form part of the common general knowledge in the art. In view of the common general knowledge in the art, it can be readily concluded that, in substance, the invention in claim 3 amounts to a mental method performed by a computer. Following 22.02.01, the addition of a computer does not make a non-statutory method statutory. Having determined that no statutory subject-matter has been contributed, the defect associated with claiming a mental method is identified under section 2 of the Patent Act.
Claim 4 defines a computer capable of performing the method of claim 3. For it to be patentable, some technological advance would have to have been made in the operation of the computer itself. The claim defines “an input means for inputting”, “a processor means for searching ... and calculating” and “an output means for displaying”. These are the discrete statutory elements of the system and represent hardware and software components capable of performing the stated functions. The remaining features of the claim pertain to what values are to be inputted, looked up, used in the calculations and displayed. These features have purely intellectual significance and do not define how the system is operated as a technological entity. As drafted, it is self-evident that the technological functionality required of the defined statutory means is present in a general purpose computer. The claimed matter lacks novelty in view of the common general knowledge in the field of computers and does not comply with section 28.2 of the Patent Act. The claim can also be considered defective under section 2 of the Patent Act for attempting to distinguish over known subject-matter by features having a solely intellectual significance.
Date modified: October 2010
An invention must be useful, in the sense of doing whatever was promised by the inventors. The utility of the claimed subject-matter must be established by demonstration or sound prediction, and this subject-matter must be operable to produce the promised result in a manner that is controllable and reproducible.
A computer is generally considered to be capable of reproducibly performing whatever operations its hardware and programming enable. The utility of a computer-implemented invention is not guaranteed by this fact, however. Even where the components of the computer are working as intended, the invention as a whole may require other elements for its proper operation.
Where the judgement or interpretative reasoning of an operator is implicated in the proper operation of the claimed invention, such as deciding on suitable computer-managed operations through the exercise of judgement and reasoning, the criterion of reproducibility will not be satisfied. Where an operator’s input is required, but there is no judgement associated with the input, the need to rely on the input does not cause a lack of reproducibility [see section 19.01.01 of this manual].[286]
Where a computer-implemented method is being claimed, it must be unambiguously clear which steps of the method are being carried out on or by a computer [see 22.08.01].
Date modified: October 2010
The general requirements for a sufficient disclosure of an invention are detailed in Chapter 14 of this manual, and apply equally to computer-implemented inventions as to any other.
Certain aspects of a correct and full description of a computer-implemented invention warrant particular attention, and are discussed in the following sections.
Date modified: October 2019
In accordance with subsection 27(3) of the Patent Act, the specification must correctly and fully describe the invention. In practice, this requirement relates to the description, which must support the claims in accordance with section 60 of the Patent Rules.
The two requirements of a description are i) that it disclose in clear and unambiguous terms the nature of the claimed invention (written description requirement) and ii) that it provide any teachings necessary to allow a person skilled in the art to operate the claimed invention (enablement requirement). A person skilled in the art must be able to understand, in view of the specification alone when read in light of their common general knowledge, what the invention is, what it does, and how to make it work.
The level of description necessary will depend on the facts of each case. In general, where aspects of common general knowledge are referred to, it may not be necessary to do more than identify a well-known element or technique forming part of this common stock of information. Where specific information is required that does not form part of the common general knowledge, this must be explicitly provided. For example, if certain hardware and software are known in the art at the date of invention, it will be obvious that they can be used to achieve known or predictable results or perform known or predictable operations. It may be possible to describe and enable those aspects of the invention that relate to this known hardware or software simply by identifying the particular hardware or software element to be used and the known or predictable result to be achieved. In contrast, if the desired result requires a novel and unobvious application of hardware or software, a greater level of detail regarding how this result is to be achieved would be necessary.
Where a claim defines the invention in terms of means-plus-function statements, the nature of the means, and where applicable how they are arranged to provide the stated functionality, must be clear to the person skilled in the art. The level of description necessary to correctly and fully describe the means, and their arrangement where applicable, will depend on the state of the common general knowledge in the art.
Where limited description is provided, this is taken as an indication that the applicant (rightly or wrongly) considers that the selection of suitable means to perform the stated function would be readily apparent to a person skilled in the art.
Computer-implemented inventions are often described in terms of a flow chart that illustrates the algorithm or logic tree on which the operation of the invention is based. Typically, the flow chart will set out the operations performed by a computer. Flow charts are diagrams having a series of boxes, each representing a state or a step in an algorithm, and arrows that interconnect these boxes to describe the order or relationship of the various steps.
It will often be the case that the algorithm or logic performed by the computer lie at the heart of the invention. In such circumstances, a full description of the algorithm or logic tree should be provided. Where the algorithm or logic is described by reference to a flow chart, presented as a drawing, a written explanation of the flow chart is necessary to provide support for any claims that refer to the algorithm or logic.
In order to successfully practice the invention, it is necessary for the person skilled in the art to be able to put each step in the flow chart into operation. For the description to be enabling, the person skilled in the art must be able to do this without recourse to inventive ingenuity or undue experimentation. The flow chart, and any accompanying description, must therefore provide any information necessary to enable the algorithm to be so practised.
The amount of written description necessary to properly describe and enable an algorithm depends on the relationship of each step to the common general knowledge. Where the algorithm invokes well-known operations, it may be that very little or no specific description is necessary for the purposes of proper description or enablement. If, in contrast, the specific operations necessary to enable a step in the algorithm would not be obvious to the person skilled in the art, these operations would need to be fully described.
Furthermore, if the common general knowledge of the person skilled in the art would lead them to attempt to enable the algorithm in ways that would not in fact work, the description should provide sufficient instructions to allow the person skilled in the art to arrive at operable embodiments and avoid inoperative ones.
Where very little explanation is given regarding how a step in a method is to be implemented by a computer, this will generally be understood as an indication that the applicant, rightly or wrongly, does not consider the implementation of that step to require inventive effort on the part of the person skilled in the art.
Date modified: October 2010
Source code or pseudocode may be provided as part of the description of a computer-implemented invention, but will generally not be considered, by themselves, to provide a full and enabling description of an invention.
Where source code is provided, it must be remembered that the significance of the commands used in specific code may depend on the intended platform, and the code itself will generally not be a clear and unambiguous description of the invention.
Pseudocode refers to a semi-structured, natural language explanation of the functioning of an intended program, and may be used as an alternative to a flowchart to provide a set of instructions with a logical sequence but which do not follow the syntax of any particular programming language. Pseudocode will therefore usually have a greater value in describing an invention than source code in a specific programming language. However, in the same way that a flowchart will usually require an accompanying description in order to fully describe an invention, pseudocode alone will typically not be sufficient to provide a full and unambiguous description of an invention.
Date modified: October 2010
The activities required to reduce a specific series of logic instructions to a computer code are considered to form part of the common general knowledge of a skilled programmer. It is, therefore, typically not necessary for an inventor to describe how to write computer code, either in general or in respect of a specific computer language.
Where the algorithm to be written out as lines of code only invokes well-known operations, or if specific and unobvious logic operations are required, where these have been clearly described, the act of expressing the specific commands as lines of code is considered not to require inventive ingenuity or undue effort.
Where the description only discloses in broad terms what the program is intended to do, and it would not be clear to the person skilled in the art in view of their common general knowledge what the required operations are or the logic necessary to enable specific required operations, then the skilled programmer has not been given sufficient instructions to create the necessary code. To create a working program, the programmer would first have to exercise ingenuity in order to solve the problem of reducing the concepts disclosed to a series of practical instructions (i.e. would need to design the program; see 22.03.01).
Date modified: October 2010
As with every invention, in order to be patentable a computer-implemented invention must not be anticipated by prior art that is relevant under section 28.2 of the Patent Act.
To be anticipatory, a single prior written disclosure, when understood in light of the common general knowledge, must both provide a written description of the claimed invention and sufficient instructions to enable the invention to be practised by the person skilled in the art without recourse to inventive effort or undue burden.
In considering whether a claimed invention is anticipated, its essential elements must be compared to those taught in a single prior disclosure. If all its essential elements were previously disclosed, the invention is anticipated. The essential elements of an invention are those that have a bearing on what the invention will do and how it does it (i.e. on its practical and promised utility).
When considering a computer device (machine) claim, the effect of any commands being implemented by software must be carefully considered in order to determine if they lead to a technological effect relevant to the promised utility of the device. If so, those commands are essential elements of the device, and must be considered during the novelty analysis. If the commands are simply an application of functionality the machine was already known to possess, they are not considered to be essential elements of the machine itself.
Date modified: October 2010
Although the majority of prior art consists of prior written disclosures, a prior sale or use of an invention can also amount to an anticipation, provided it makes available information which describes the claimed invention and amounts to an enabling disclosure.[287]
With regard to computer-implemented inventions, software that was available to the public prior to the claim date can be considered as prior art. To be considered to have disclosed the claimed invention, the software must provide to the person skilled in the art information sufficient to comprehend the invention.[288] The use of a product makes the invention part of the state of the art only so far as that use makes available the necessary information.[289] The information made available must be such that if the person skilled in the art were to write down that information, they would have drafted a clear and unambiguous description of the claimed invention.[290]
Thus, if the claimed invention is defined broadly using functional language, any prior art software that achieves the same function could be anticipatory. In contrast, if the claimed invention defines a particular method for arriving at a specific result, prior art software would only be anticipatory if it could be established, on the balance of probabilities, that it was using the same method for arriving at the result.
As was noted in Baker Petrolite Corp. v. Canwell Enviro-Industries Ltd., in determining whether a publicly available product anticipates a claimed invention, the ability of the person skilled in the art to reverse engineer the product “in accordance with known analytical techniques” may be relevant.[291] Therefore, where relevant, the ability of the person skilled in the art to reverse engineer software, without inventive effort, in order to ascertain what method it implements must be considered. Note that what is considered is the ability to reverse engineer, such as by decompiling; it is not necessary to establish that the product was actually reverse engineered.[292]
In considering whether anticipation by prior sale or use of an invention has occurred, the grace period provided for in paragraph 28.2(1)(a) of the Patent Act applies in respect of any making available of the invention by the applicant or by a person who obtained the relevant knowledge directly or indirectly from the applicant.
Date modified: October 2010
As with every invention, in order to be patentable a computer-implemented invention must not be rendered obvious by prior art that is relevant under section 28.3 of the Patent Act.
Obviousness is evaluated in view of the overall state of the art contained in the prior art, when this is considered as a whole in light of the common general knowledge of the person skilled in the art. A claimed invention must be the result of ingenuity, and a conclusion of obviousness is equivalent to a conclusion of lack of inventive step. To be considered obvious, the teachings present in the prior art must be sufficient so that, if combined, they would lead to the claimed invention. Furthermore, it must be uninventive (obvious) to combine the necessary teachings.
As with the assessment of novelty, the assessment of obviousness is based on the essential elements of the claimed invention. There is nothing inventive in adding a non-essential element to an invention, since by definition the non-essential element is irrelevant to the invention’s successful operation.
It is considered obvious that computers can be used to automate many manual operations, and the idea of automating a manual process is, in the absence of reasons to conclude the contrary, considered to be uninventive. The inventive step necessary to support a claim to a computer-automated version of a known manual method therefore must typically be found in the solution to specific challenges attendant to enabling the automation.
Where a computer-implemented invention aims to achieve a new unitary result through the use of a combination of known hardware and software, an inventive step may exist by virtue of the recognition that the combination will achieve that result. If, in contrast, using the hardware and software together merely results in a predictable outcome, the alleged invention is a mere aggregation.
Date modified: October 2019
A computer-implemented invention is typically claimed as a machine, a method (an art or process) or a manufacture (computer-readable medium). As with any type of claim, a claim to a computer-implemented invention must meet the requirements of, inter alia, subsection 27(4) of the Patent Act and section 60 of the Patent Rules.
Date modified: October 2010
Where a claim is directed to a method that is to be implemented in whole or in part by computer, it must be unambiguously clear which steps of the method are being carried out by the computer.
Specifying in the preamble that a method is “computer-implemented” implies that some, but not necessarily all, steps of the method are performed by a computer. Where, in view of the specification as a whole, a given step can be understood as being performed either by a computer or by a person, it should generally not be presumed that the claimed method requires that step to be performed by a computer.
Date modified: October 2010
Where a claim is directed to a machine, it must be defined in terms of physical components.
Many computer claims will define the device in terms of means statements that set out what the device will do. Where a means statement is understood to be a software means, it must be specified that the software is stored on a physical memory. This can be done in the claim itself or in the description, with due regard being given to the need for the language of the claim to be clear, concise and unambiguous.
In some cases, it is possible that the means referred to in a means statement can be either hardware or software. In such cases, it may be most convenient to specify in the description that the means statement refers to either hardware or software on a physical memory.
Date modified: October 2010
The term system, depending on the context in which it is used, may refer to a machine (a device or apparatus, or a network of devices or apparatuses), a computer program or set of computer programs (e.g. a database management system or an operating system), or a method. Consequently, care must be taken to ensure that its intended meaning in a given context is unambiguous.
In the computer arts, where it is not clear that something else is meant it may be presumed that the term system refers to a machine.[293]
Regardless of which meaning is intended, it must be clear which category of invention the claimed subject-matter is meant to belong to. Where the claimed system is not a machine, it may be necessary to explicitly define that it is, for example, a software product or method in order to comply with subsection 27(4) of the Patent Act.
Date modified: October 2010
A computer program (software), when claimed per se, is considered by the Office to be an abstract scheme, plan or set of rules for operating a computer [see section 17.03.08 of this manual], and consequently not to be an invention within the meaning of section 2 of the Patent Act.
Under certain circumstances, software can be claimed by directing the claim to a physical memory storing the computer program. A claim to a physical memory falls within the category manufacture.
In defining a software product, the form of the claim is important. The preamble must clearly direct the claim to a physical product limited by the computer program stored thereon, and not to a computer program limited by having been stored on a memory. Thus, the preamble “a physical memory having stored thereon...” directs the claim to a statutory embodiment, whereas “a computer program stored on a physical memory” directs the claim to a computer program and thus to excluded subject-matter.
Furthermore, it must be explicitly defined that the computer program is present as machine-executable code. Only machine-executable code can change the technological functionality of the physical memory storing the program. Non-executable code is considered to be mere descriptive matter [see section 17.03.06 of this manual].
Where the computer program would cause the device it controls to provide a technological solution to a technological problem, the “software-modified physical memory” is a single discrete element. Where the program is novel and inventive, the claim will include a statutory contribution. These, then, are the circumstances under which a software product comprising a physical memory storing executable code can be patented.
Example:
An application is directed to a computer-implemented method for determining a channel assignment in a Code Division Multiple Access (CDMA) network. The method improves CDMA networks by determining CDMA channel assignments according to predetermined constraints. It has been discovered that appropriate predetermined constraints improve efficiency in the network.
The prior art search reveals that the following features were known from D1:
D1 does not disclose the use of predetermined constraints to modify channel assignments
Claims:
Analysis: Claim 1 defines a technological method comprising physical steps, and is therefore statutory in form. Assigning channels in a CDMA network according to the method results in an improved communications network; the method therefore provides a technological solution to a practical problem and the steps pertaining to the predetermined constraints are technologically distinct from similar steps performed without the constraints. The prior art does not disclose the feature of using predetermined constraints to modify an initial channel assignment in a CDMA network. Presuming that the examiner determines this to be an inventive feature, at least one physical step in the method will have been contributed. The claim would then include a statutory contribution and be allowable. Note that, to avoid indefiniteness, it would be necessary in an actual claim to define the actual “predetermined constraints” being relied on.
Claim 2 defines a computer program per se and is therefore directed to non-statutory subject matter by its form. The claim is objected to under section 2 of the Patent Act.
Claims 3 and 4 are alternative ways for defining a computer product. Both are acceptable in their form. To be patentable, the physical memory must be considered to be technologically distinct from other physical memories. This is considered to be the case where the computer program stored on the memory would cause a computer running the program to itself be a technological solution to a technological problem. A computer programmed in a novel way to implement the entirety of an inventive method is patentable in its own right. Where the programmed device would be patentable, a physical memory storing the program as computer executable code is also patentable. Therefore, where the method of claim 1 would be patentable, either of claim 3 or claim 4 would also be allowable.
Date modified: October 2010
A “means” statement defines some part of an invention in terms of a means suitable for achieving a result, rather than by explicitly defining those specific things that would yield the result. Means statements are not objectionable per se, provided the claim meets all the requirements of the Patent Act and Patent Rules.
In order for a means statement to be properly supported, the description must describe what types of means are contemplated by the inventor unless this would be obvious to the person skilled in the art in view of their common general knowledge. Where it would not be obvious to the person skilled in the art which means fall within the scope of a defined means statement, the claim may be defective for lack of proper support or for indefiniteness. A means statement may refer to hardware or to software, and it should be clear in the context of the claim what the means statement refers to.
In the computer arts, the term “means” is often used in reference to a computer running software. Unless the context of the claim precludes this interpretation, a means statement that encompasses software may be understood to refer to software stored on a physical memory and being executed by a processor.
Date modified: October 2010
The subject-matter of a claim must belong to a category of invention as defined in section 2 of the Patent Act. The elements used to define the subject-matter must consequently be of a type appropriate to that category of invention.
Where a claim in one category of invention (e.g. a machine) defines its subject matter in terms of elements from another category (e.g. method steps), there is a risk of ambiguity over the intended subject-matter.
Where a claim is directed to a machine, it must define its subject-matter in terms of structural components whereby the machine can be distinguished from all other machines. Given that computers are often defined in terms of means statements that provide functional limitations to the machine, care must be taken to ensure these means statements can be understood to be physical components [see 22.08.02].[294]
Where a claim is directed to a method of using a device, it must include at least one step whereby the device is applied to the task at hand. A claim simply reading “A method of using the device of claim 1.” may be considered indefinite, for example, since the manner by which the device is used has not been defined.
Note that the “product-by-process” claim type defines a product wholly or partly in terms of the process by which it is produced. It is not a format for defining a product in terms of the method for which it will be used.
Date modified: October 2010
This section addresses specific types of subject-matter for which particular attention, elaboration or clarification was considered appropriate.
In the following sections, the example claims are analysed following the approach set out in Chapter 12. Furthermore, the analyses focus primarily on the question of whether a statutory contribution exists on the presumed facts of each example. In attempting to provide simplified examples, little consideration has been given to the question of enablement. Many of the example claims are defined in terms of broad functional statements (“means for” statements). In practice, whether these are properly supported would depend on the degree of disclosure and on the common general knowledge in the field [see section 22.05].
Date modified: October 2010
A “Graphical User Interface” (GUI), as the name implies, refers to a type of interface for enabling a user to interact with a computer or a computer-based device. While early computers used command line interfaces that required the user to enter textual commands to control a computer, graphical user interfaces enable the user to interact with the computer via visual elements such as icons, buttons, menus, toolbars and other graphical screen elements.
The term GUI is considered by the Office to refer only to the arrangement of visual elements that will be displayed on a screen, and not to include any of the hardware or software components that may be required to generate the graphical user interface or to make it functional. A GUI as such is consequently considered to be information, that when displayed on a screen is subject to the practice set out in section 17.03.06 of this manual.
An invention is considered to be a solution to a practical problem, which the Office considers to imply a “technological solution to a practical problem”. Features having purely intellectual or aesthetic significance are not statutory subject matter and cannot provide a statutory contribution. Any display of information wherein the sole contribution is in the information itself amounts to non-functional descriptive matter, and is not a patentable contribution [see section 17.03.06 of this manual].
The specific arrangement of graphical elements on a screen, or in other words the visual design that defines a graphical user interface, is viewed by the Office as not constituting a patentable contribution where the visual design of the graphical user interface does not provide a technological solution to a practical problem. Rather, it is viewed as having purely aesthetic significance and amounts to non-functional descriptive matter.
However, the presence of a graphical user interface does not exclude an invention from patentability if the criteria for patentability are satisfied. A GUI that has been integrated with statutory subject matter may be patentable. Claims including a GUI must be directed to one of the categories of invention, as defined in section 2 of the Patent Act.
Example 1:
An application discloses a portable device that allows a user to read an electronic book. The device comprises a touch screen, and displays the electronic book using an efficient graphical user interface that provides buttons for frequently used operations at the top of the screen, hyperlinks to other content within the book on the left of the screen, and a central frame for displaying the content of the book. The device also allows the user to enter personal notes at any location within the content of the electronic book. The personal notes are stored within XML tags that are embedded within the content, and a graphical icon is displayed at the location of each XML tag. The user is able to view stored personal notes by clicking on the relevant graphical icon. The touch screen is able to recognize advanced user touch commands, and the device comprises software to interpret such touch commands and perform specific functions.
The prior art search reveals that the following features are known from D1:
The prior art does not disclose the efficient GUI arrangement of this application, the feature of storing personal notes using XML, or the feature of recognizing advanced touch commands.
Claims:
Analysis: Claim 1 defines a graphical user interface per se and is therefore directed to non-statutory subject matter by its form. The claim is objected to under section 2 of the Patent Act.
Claim 2, in contrast, is directed to a device and is therefore not objectionable in terms of its form. Upon closer examination, it is evident that claim 2 contains both statutory and non-statutory features. The portable device and the touch screen are two statutory features, while the arrangement of screen elements as defined in the claim is a non-statutory feature. The touch screen provides a technological limitation to the portable device, so the two are considered to be a single discrete element of the claim. However, the arrangement of screen elements does not provide a technological limitation to the portable device having a touch screen, and is therefore considered to be a second discrete element of the claim. In order to determine if the subject matter of claim 2 includes a statutory contribution, the prior art features disclosed in D1 must be compared to the statutory discrete element recited in the claim. Given that the prior art discloses a portable electronic book reading device having a touch screen, this feature does not form part of the contribution of the claim. It is not necessary to assess whether the arrangement of screen elements has been contributed, since it is a non-statutory discrete element and cannot itself result in a statutory invention. Following the contribution analysis, it is determined that claim 2 does not contain a statutory contribution. An objection under section 2 of the Patent Act on the basis of the non-statutory subject matter would be appropriate, since this matter is the point of the invention.
Claim 3 defines a computer program on a physical medium. The software allows the GUI of claim 1 to be displayed. The claim does not define any features that define a technological solution to a technological problem. The GUI of claim 1 remains a discrete element of the claim, and the physical memory comprising software that enables information to be displayed is a second discrete element of the claim. It is clear from D1 that software for displaying information was known in the prior art, and the memory having such software stored on it is therefore not part of the contribution. The claim can be objected to in the same manner as was claim 2.
Claim 4 is again directed to a computer program on a physical medium, but recites additional features allowing the user to embed personal notes at specific locations within the content of the electronic book using predefined XML tags, and to subsequently display the personal notes upon request. These features work together to modify the way in which the device executing the instructions stored on the computer readable medium operates, in such a way that they provide new functionality to solve a practical problem. In this case, the practical problem being how to enable the user to store and retrieve personal notes at specific locations within the content of an electronic book. Since the device itself would provide a technological solution to a technological problem and would be considered statutory, the computer readable medium storing the instructions that would control the device is also considered to be statutory [see 22.08.04]. If the examiner determines, based on the state of the art at the claim date, that the feature of embedding notes within the content of an electronic book using XML tags is novel and inventive, then this would be regarded as a statutory contribution and the claim would be allowable.
Claim 5 recites an additional feature of recognizing a specific touch command performed by the user of the touch screen, and performing a specific functionality based on such a touch command. Although the prior art touch screen allowed the user to point and click, it did not have the ability to recognize a complex motion such as a pinching motion similar to how a person would flip a page in a physical book. This feature is regarded as a technological feature providing new functionality to solve a practical problem, which is in this case to provide functionality to the touch screen to enable the user to conveniently browse through an electronic book using normal hand gestures. Since this feature is a technological modification to the portable electronic device, the overall modified device is now considered to be a single discrete element. If the examiner determines that this functionality is novel and inventive, a statutory contribution would be present in the claim and it would be allowable.
Example 2:
An application discloses a system for controlling the operation of network devices. Each device stores self-describing information detailing what type of device it is, and what control options are available to network users. A graphical user interface displays unique icons representing each device on the network, as well as a customized menu for each device showing available control options. The unique icon and the available control options are retrieved from each device on the network dynamically, resulting in a graphical user interface that accurately reflects the network at all times, even when changes are made to the network or the network devices.
The prior art search reveals that the following features were known from D1:
The GUI of D1 is static and does not obtain self-describing information from the devices.
Claims:
Analysis: Claim 1 is directed to a GUI, and further defines that the GUI is generated by a computer program and that program will dynamically retrieve certain information from devices attached to the computer. The claim is directed to excluded subject-matter by its form, however, and is objected to under section 2 of the Patent Act. The presence of the computer program feature indicates how the GUI is generated and modified, but the claim itself is still directed to a GUI per se.
Claim 2 is directed to a computer-implemented method wherein graphical elements are displayed and wherein the content of the display is dynamically updated by the computer program that generates the GUI. This method of controlling the operation of the computer provides a technological solution (dynamic querying) to the practical (technological) problem of having a current list of control options available for each peripheral device attached to the computer. The method enables the graphical user interface to be dynamically updated as devices on the network are added, removed or modified, and results in a more efficient system for controlling network devices. The method is statutory in form. Each step in the method includes both a statutory discrete element (displaying graphical elements or dynamically retrieving information) and a non-statutory discrete element (the information that is displayed or retrieved, and which does not limit the technological aspects of displaying or retrieving). The statutory steps of displaying graphical elements and dynamically retrieving information from the peripheral devices would be examined to determine if the overall method is both novel and inventive over the prior art. Since the steps operate together to provide a unitary result, they are compared to the prior art in combination.
Note that if the method is considered to be novel and inventive, a claim to a device operating the method or to a physical memory storing the software that enables the method would also be allowable.
Date modified: October 2010
A data structure is a format for organizing and storing a collection of related data items to suit a specific purpose. A particular data structure may enable or facilitate a specific set of operations to be performed on the data items easily and efficiently, for example to improve the performance of computer programs and minimize the consumption of computer resources. Examples of data structures are arrays, records, linked lists, stacks and trees.
The Office considers a data structure to be an abstract idea or plan for organizing data items, and not to include the physical medium upon which the data structure is to be stored. A data structure per se is consequently considered to be disembodied and not an invention within the meaning of section 2 of the Patent Act [see section 17.02 of this manual]. For a data structure to have an impact on the patentability of a claimed invention, it must in some way limit the technological nature of a statutory element in the claim.
Example:
An application discloses a networking system that guarantees a quality of service for a networking connection, wherein the system comprises networking equipment that is used to transmit data packets across a network. The data packets include a quality of service indicator that is read by other networking equipment along the path of the transmission, such that the networking equipment will prioritize delivery of packets with a higher quality of service guarantee.
The prior art search reveals that the following features are known from D1:
The prior art does not disclose prioritizing packet delivery based on a quality of service indicator within the packet header.
Claims:
Analysis: Claim 1 defines a data structure per se, and is therefore directed to non-statutory subject matter by its form.
Claim 2, in contrast, is directed in form to a physical memory, and consequently to a statutory manufacture. The data stored on the memory does not alter the technological character of the memory, and therefore is a discrete element of the claim. The claim, consequently does not include a statutory contribution. Since the data structure is the point of the invention, an objection could be presented under section 2 of the Patent Act on the basis of a contribution analysis. Note that the conclusion differs from that which could be reached if the physical memory were storing executable computer code that made use of the structure to render a computer more efficient or reliable.
Claim 3 defines a method for transmitting and receiving data wherein the system can prioritize data based on its quality of service indicator. The data structure is made use of to control the manner by which data packets are transmitted, and this changes the technological character of step b). The step of prioritizing delivery is understood to involve an analysis of the packets, an evaluation of network traffic and available bandwidth, possibly storing certain packets temporarily, etc. Depending upon the state of the art and the common general knowledge in the field, such details might need to be defined in an actual claim. Both steps in the method are technological in nature, and the method provides a technological solution to a practical problem and is statutory. If the data structure and its technological effect are found to be novel and inventive, the method would be patentable.
Date modified: October 2010
In general terms, a database refers to a collection of information organized so that it can be stored, searched and retrieved easily. Computer databases can be implemented in many forms, the simplest being to store information in a text file in a specific format (a data structure) to enable the information to be subsequently retrieved. More advanced implementations employ specialized software, often referred to as a database management system, to control access to the stored information. Examples of common database management systems in use today include Microsoft™ Access™, MySQL™, and Oracle™.
The Office interprets a database to be solely a collection of information, and not to include the physical medium upon which the database is stored. A database per se is consequently considered to be disembodied and not an invention within the meaning of section 2 of the Patent Act [see section 17.02 of this manual]. Where a database, as a feature of a claim, limits the technological nature of a statutory element in the claim it can result in a statutory contribution.
A database management system is generally understood in the art to be a computer program [see 22.08.03 on system claims]. A claim to a database management system computer program is not directed to a statutory invention whereas a claim to a physical memory storing a database management system defines, in form, a statutory manufacture [see 22.08.04].
Example:
An application discloses a distributed database system to reduce the load on database servers in a network. The same database is stored on multiple database servers. A common control server receives database access requests and distributes them among the multiple database servers. The control server keeps track of the load on each database server, and distributes requests in order to evenly distribute the load on the servers. The control server also periodically synchronises the data across the database servers during periods of lighter load, in order to maximise performance of the overall distributed database system. The application describes the use of the distributed database system for a web based social networking application.
The prior art search reveals that the following features are known from document D1:
The prior art does not disclose the feature of a common control server keeping track of the load on the database servers in order to evenly distribute access requests and scheduling database synchronisation during periods of light server load, which results in improved performance of the overall distributed database system.
Claims:
means for distributing received database access requests among the plurality of database servers; and
means for performing database synchronisation to synchronise the content of the databases stored on the database servers.
· wherein the distributed database is used to store for each user of the application:
· account information;
· profile information;
· a list of relationships between users; and
· messages sent and received by each user.
and wherein the database synchronisation is performed during periods where the database servers are experiencing a lighter than normal load.
Analysis: Claim 1 defines a plurality of servers i), wherein each server stores a copy of the database, and a control server ii) which comprises means to manage the system as a whole. The means statements are understood to be software stored on a physical memory and executed by the server’s processor. The means both alter the technological operation of the control server ii), and the “software on a physical memory” means are therefore statutory elements of the claim. Equivalently, the “means-modified control server” may be considered a single discrete element of the claim. Each server i) is also a discrete element of the claim, as is the database (which does not provide a technological limitation to the server storing it). The patentability of the claim will depend on whether server ii) is found to be novel and inventive, since the servers i) are known and since the database is not a statutory feature of the claim. In view of D1, the server ii) would be considered novel. For the sake of this example, it is presumed that the server is found to be obvious in view of the cited prior art and knowledge in the field. The claim would therefore be objected to under section 28.3 of the Patent Act.
Claim 2 adds to the features of claim 1 a web-based social network application server, and defines the information stored for each user of the system. The application server is a statutory feature. In this example claim, there is insufficient information defined about the nature of any software on the server (i.e. how the social network application works) to determine whether the software would enable the server to solve a particular technological problem. In view of D1, which discloses a web-based application, it does not appear that the server iii) distinguishes the system over the prior art. The further feature of the claim, the specific information stored, is a non-statutory feature which does not provide a technological limitation to the server. The data is therefore a discrete element of the claim. To the extent it appears the applicant is asserting the data in order to distinguish the invention, an objection under section 2 of the Patent Act, referring to a contribution analysis, is warranted.
Claim 3 adds to claim 2 the additional feature of the system comprising means for tracking the load of the database servers, distributing database access requests according to this information in order to evenly distribute the load on the servers, and performing synchronization during periods of lighter than normal load. This means is, again, understood to be software stored on a physical memory and being executed by a processor. The means provides new technological functionality to the control server, and is a statutory “software on a physical memory” element of the claim. Equivalently, the means-modified server can be considered to be a single discrete element of the claim. If the examiner considers that the server having a means to provide the defined functionality is novel and inventive over the prior art, claim 3 would be considered to involve a statutory contribution and would be allowable.
Claim 4 defines a database per se, and is therefore directed to non-statutory subject matter by its form. The examiner will object to this claim under section 2 of the Patent Act.
Date modified: October 2010
A computer-aided design program is a computer program specifically used in the design of objects and to perform simulations on designed objects before the final product is actually built, thereby leading to significant reductions in time and cost. CAD programs are used in many industries including architecture, automotive, electronics and computer animation among others.
CAD programs are typically not capable of independently performing the act of designing; rather they are tools that are used by designers to help with the design process. Inventions related to CAD programs will therefore usually focus on the functionality of the CAD program as a tool used to assist the designer, and not on their ability to independently carry out a design. While methods of designing may be viewed as schemes or mental processes, which are disembodied and not a practical form of an invention, CAD programs are tools that are used during the design process and may comprise a technological contribution.
A CAD program is a specialized type of computer program, and consequently the practices pertaining to computer programs apply to CAD programs.
Example 1:
An application discloses a computer-aided design tool for automatically performing integrated circuit placement, layout and routing. The tool starts its process by reading a netlist file defining all the components in a circuit schematic and their interconnections. The CAD program then performs the circuit placement, layout and routing using a hierarchical approach wherein simple circuit cells (sub-circuits within the overall circuit) are optimised first (this being the lowest level in the hierarchy), then larger sub-circuits (second and subsequent levels in the hierarchy), and so on until the overall circuit has been created. The program first scans the circuit to look for circuit cells, optimizes one example of each such cell and adjusts all others according to the optimized result. It then scans the circuit looking for larger cells and repeats the process until the overall circuit has been optimized. Since each higher level is optimised relying on the results of the lower level optimisation, fewer operations are needed overall in order to optimise the overall circuit. The approach also avoids “false minimum” optimisation results that can occur when the starting point of the optimisation is too unrelated to the actual optimised circuit. The optimised circuit can be displayed as an image, schematic, or as a control file for a computer-controlled fabrication process.
The prior art search reveals that the following features are known from D1:
The prior art does not disclose using a hierarchical approach to perform the layout and routing.
Claims:
a. performing integrated circuit layout of the individual circuit cells;
b. identifying the interconnections between the circuit cells;
c. performing placement and routing of the circuit cells while minimizing interconnection length and routing complexity;
Analysis: Claim 1 defines a computer-implemented method whose object is the solution of a technological problem – how to provide an optimised layout of a circuit based on predetermined input parameters while avoiding “false minimum” results and minimising the number of operations necessary to optimise the circuit. The method as a whole therefore is statutory in its form. Each step in the method involves a series of computer operations for performing a specific task. The steps of reading the netlist file and generating an output file can be treated as discrete elements, since they do not limit the technological nature of the remaining steps. They represent known computer operations and are presumably not part of the contribution.
For this example, it is presumed that the hierarchical approach to optimising the circuit was not previously known and would not be obvious. The method provides a technological solution to a practical problem in the operation of the computer: it requires fewer computer operations to arrive at the optimised circuit than the prior art method, and in effect allows the computer to perform the optimisation more accurately and efficiently. The steps in the method relating to how the computer performs the analysis are therefore a statutory contribution, and the claim is consequently patentable.
Note that the question of how the hierarchical analysis and optimisation is performed is essential to the claimed invention; it is worth reiterating, in respect of this example in particular, that depending on the extent of the description and the state of the common general knowledge, specific details regarding the implementation of the method may be required in the claim.
Note that if the hierarchical approach had already been known, the analysis would be different. In that case, a contributed technological solution to a technological problem would only exist if a specific obstacle to implementing the steps relating to the hierarchical approach in a computer had been overcome. In such a case, the specific inventive operations to be performed by the computer to provide this solution would need to be specified.
Claim 2 is directed to a computer program per se, and is defective in form.
Claim 3, in contrast, illustrates a claim properly directed to a computer product. Given that the method of claim 1 is patentable, a computer implementing the entire method also would be patentable. The subject-matter of claim 3, a physical memory embodying a computer program that would render a computer running it patentable, is likewise patentable.
Example 2:
An application discloses a CAD program for optimizing transistor sizing for combinatorial networks. The program uses the Logical Effort gate delay model to optimize transistor sizing based on gate load and the desired delay characteristics. The program takes as inputs a schematic netlist file and the desired delay through the critical path of the circuit. The program calculates the optimum width and length for each transistor in the critical path of the circuit, and produces an output netlist file with that information.
The prior art search reveals that the following features are known from D1:
The prior art does not disclose using a computer program to automatically optimize transistor sizing based on Logical Effort, taking as inputs only the netlist and the desired delay.
Claims:
Analysis: Claim 1 defines a method for using a computer to optimise a circuit schematic. The claim is statutory in its form. The steps of reading a netlist file and generating an output netlist file can each be considered a discrete statutory element of the method, and it is understood that neither forms part of the contribution.
The remaining steps relate to a series of calculations. It is presumed for the purposes of this example that the description does not disclose any obstacles that were encountered in implementing the calculations on the computer. The sequence of operations necessary to perform the calculations would have been self-evident to a person skilled in the art presented with the equation. Consequently, there was no technological innovation in enabling the computer to perform the calculations. The steps of calculating are consequently simply the performing of an otherwise non-statutory method of calculation on a computer. Absent a technological problem to be overcome in how the computer performs the calculations, there is no statutory contribution in the claimed matter. Given that the specification emphasises the importance of the specific calculations, it would be appropriate to object to the claim under section 2 of the Patent Act in light of a contribution analysis.
Date modified: October 2010
The Office regards electromagnetic and acoustic signals and waveforms to be forms of energy and not to contain matter despite that the signal may be transmitted through a physical medium. As a result, claims to electromagnetic and acoustic signals do not constitute statutory subject-matter within the definition of invention in section 2 of the Patent Act.
More particularly, an electromagnetic or acoustic signal is interpreted to be neither an art nor a process because it is not an act or series of acts or method of operation by which a result or effect is produced by physical or chemical action. Neither is an electromagnetic or acoustic signal a machine, as it is not the mechanical embodiment of any function or mode of operation designed to accomplish a particular effect, or a composition of matter, as it is not a chemical compound, composition or substance. An electromagnetic or acoustic signal is considered not to be a material product and, therefore, not a manufacture.[295]
The Office considers signals to be transitory in nature, and to exist only while being propagated.[296] Once the information contained in a signal has been stored on a physical medium, it is no longer considered to be a signal and is more appropriately referred to as data. Therefore, claims that define a physical medium storing a signal or a waveform are considered indefinite under section 27(4) of the Patent Act. See section 17.03.04 for further information.
Although signals per se are not patentable, methods, processes, machines or manufactures involved in the generation, transmission, reception, or processing of signals may be patentable if all other criteria for patentability are satisfied.
Example:
An application discloses a transmission system to transmit video data over short distances. The system uses a carrierless ultra wideband signal, where the video data is encoded into multi-phase wavelets. The system allows for transmission at high data rates over short distances, and can be used to transmit video from a security camera to a recording device, for example. When transmitted at low power, such carrierless transmissions do not interfere with narrowband or spread spectrum signals.
The prior art search reveals that the following features are known from D1:
D1 does not disclose the use of a carrierless ultra wideband signal where the data is encoded into multi-phase wavelets.
Claims:
Analysis: Claim 1 defines a signal per se, and is therefore directed to non-statutory subject-matter by its form and is objected to under section 2 of the Patent Act.
Claim 2 defines a physical transmission medium and is therefore directed in form to statutory subject-matter. The signal does not provide any technological limitation to the transmission medium, however, and the claim therefore includes two discrete elements (the medium and the signal). Since the physical transmission medium has self-evidently not been contributed, the claim does not include a statutory contribution. As the signal of claim 1 appears to be the inventive aspect, an objection is made under section 2 of the Patent Act in light of the contribution analysis.
Claim 3 defines, in form, a statutory device. The claim recites means for encoding, transmitting, and receiving and decoding data signals. For the purposes of this example, it is presumed that it is clear from the description that certain of the means relate to hardware components and others to software stored on a physical memory. The encoding of the data into multi-phase wavelets allows the transceiver to transmit data at a high rate while minimizing interference with other signals. Thus, the technological character of the device is modified by the software-enabled encoding. The claim does not include a discrete non-statutory element, and the patentability of the claim is evaluated on the basis of the novelty and ingenuity of all the defined elements in combination. Presuming the use of multi-phase wavelets is considered novel and inventive, the claim would be allowable.
Date modified: March 2016
This chapter provides guidance on Office practice particularly as it pertains to patent applications concerning inventions residing in the diverse field of “biotechnology”, as well as “medicinal inventions”.
The field of biotechnology can be thought of as encompassing “any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use”
.[297] Medicinal inventions, by their very nature, also interact with biological systems and encompass chemical compounds or compositions (and the preparation thereof) relating to or having therapeutic properties. It is important to note that although these descriptions offer a convenient means to label an invention, an invention may simultaneously exist in more than one field of technology.
In reading this chapter, it should be borne in mind that its purpose is to clarify, through elaboration, the application of the more generic teachings of other chapters to the examination of particular subject-matter common to biotechnology and medicinal inventions.
Nothing in this chapter should be interpreted as providing exceptions to any practice of general applicability set out in any other chapter.
Date modified: March 2016
The following subsections provide guidance for determining whether claims featuring living matter define statutory subject-matter within the scope of section 2 of the Patent Act. Section 2 requires the subject-matter of an invention to fall within one of the categories of invention, i.e., an art, process, machine, manufacture, composition of matter, or an improvement in one of the preceding categories [see Chapter 17 of this manual].
Date modified: March 2016
For the purposes of section 2 of the Patent Act, life forms have in view of the jurisprudence[298] been divided into lower life forms (statutory) and higher life forms (non-statutory), with the distinction being, in general, whether the life form is unicellular (lower) or multicellular (higher).
Lower life forms are generally deemed to fall within the scope of section 2 as being either “manufactures” or “compositions of matter” since they can be produced en masse (bearing similarity to how chemical compounds are prepared) and formed in such large numbers that any measurable quantity will possess uniform properties and characteristics.[299]
Higher life forms do not fall within the scope of section 2 of the Patent Act.[300] Further, the Office takes the position that animals at any stage of development are not statutory subject-matter eligible for patent, and consequently fertilized eggs and totipotent stem cells[301] are included in the higher life form proscription.
A stem cell which is embryonic, multipotent or pluripotent[302] is not alone capable of developing into an animal and is considered to be a lower life form. Where a claim to a cell could be reasonably understood in view of the description as encompassing within its scope a fertilized egg or totipotent stem cell, this subject-matter should be expressly excluded by proviso, otherwise the claim may be construed as including matter excluded from the scope of section 2.
Note that the fact that a claimed cell could form part of a higher life form does not mean that the claim to the cell should necessarily be construed to be a claim to the higher life form. However, where, upon a purposive construction, a claim to a cell is construed to be a claim to a higher life form, the claim lacks compliance with section 2 of the Patent Act.
Where a claim is construed as an isolated cell, there is no need to specify in the claim that the cell is “as found in the laboratory” or is “in isolated form”.[303]
Lower life forms include: microscopic algae; unicellular fungi (including moulds and yeasts); bacteria; protozoa; viruses; transformed cell lines; hybridomas; and embryonic, pluripotent and multipotent stem cells.
Higher life forms include: animals, plants, mushrooms, fertilized eggs and totipotent stem cells. Plant varieties that are distinct, uniform and stable may be protected under the Plant Breeders' Rights Act, administered by the Canadian Food Inspection Agency. A plant part such as a cutting, callus, rhizome, tuber, fruit, or seed (regardless of whether the seed is coated) is also considered to be a higher life form.
A claim that is not directed to a higher life form per se but instead includes a higher life form within its scope (e.g., as a component of a composition or food product, as a use, etc.) may, depending on the essential elements as determined through a purposive construction analysis, be statutory subject-matter. For example, consider a claim to “an animal feed comprising X”. Where the claim is construed as the use of X for animal feed, the claim will likely be statutory regardless of whether X is a higher life form. When construed to be X or a product comprising X, the claim will be non-statutory if X is a higher life form. In cases where X is a higher life form that has been processed by significant chemical or physical modification, the claim may be construed as a manufacture within the definition of invention provided in section 2 of the Patent Act.
Note that a statutory method or process of producing a non-statutory higher life form will not change the determination that the life form itself is non-statutory.
Example 1:
In an application for patent, the inventors identify the problem to be solved as a need to provide a plant that is resistant to herbicide Q. The specification discloses a new recombinant plant and propagation material thereof produced by a process involving the transformation of a plant cell with an expression vector comprising a bacterial nucleic acid from S. hygroscopicus (SEQ ID NO:1) that confers resistance to herbicide Q.
Claims:
Analysis: Claims 2 and 7 are each construed to be directed to statutory subject-matter within the scope of section 2 of the Patent Act. In contrast, the subject-matter defined by each of claims 1 and 3-6 is non-statutory because each claim is construed to be directed to a higher life form, which lies outside the definition of “invention” as defined in section 2 of the Patent Act. More specifically, claim 1 is construed to be directed to a plant and claims 4-6 are construed to include seeds. Although the preamble of claim 3 defines a cell, it is important to note that the claim specifies the cell is in a plant. Thus, the claim is construed to be directed to an entire plant.
Example 2:
An application describes a novel transgenic pig that produces odourless manure due to the introduction and expression of a transgene (SEQ ID NO:2) in its genome.
Claims:
Analysis: Claims 1 and 3 are non-statutory. Claim 1 is construed as a fertilized ovum, which has the inherent ability to develop into an animal, while claim 3 is construed as a higher life form. Consequently, the subject-matter of both claims is non-statutory. In contrast, claims 2 and 4 are statutory. Claim 2 is construed as a composition of matter and claim 4 defines a statutory “art” when construed to be the use of the pig and not the pig itself.
Date modified: March 2016
Organs and tissues (whether of plant or animal origin) are generally not considered to be manufactures or compositions of matter for the purposes of section 2 of the Patent Act. Organs and tissues are in general created by complex processes, elements of which require no human intervention, and do not consist of ingredients or substances that have been combined or mixed together. In view of this, the Office considers that a genetically-modified organ or tissue is not statutory subject-matter.
Artificial organ-like or tissue-like structures that are distinct from true tissues and organs and that have been generated by human intervention through the combination of various cellular and/or inert components may be considered, on a case-by-case basis, to be manufactures or compositions of matter within the scope of section 2 of the Patent Act.[304] For example, functional and anatomical differences may be indicators that serve to distinguish an organ-like or tissue-like structure from a true organ or tissue.
Date modified: March 2016
The patentability of a method or process is independent of whether or not the product of the method or process is statutory. Processes to produce higher life forms, organs or tissues are not, therefore, defective on the grounds that they produce non-statutory products.
An especially important consideration is the degree of human intervention embodied in the claimed process. A process which occurs essentially according to nature, with no significant human intervention, is not patentable.[305] Thus, for example, a claim construed to be directed to a process for producing a plant solely by traditional cross-breeding techniques is not patentable (even where one of the cross-bred plants is transgenic or otherwise modified). A process that is a result of both human intervention and the laws of nature, however, is patent-eligible subject-matter where at least one step of human intervention is an essential element of the claim.
Processes that are considered to include significant human intervention include: processes to produce a lower life form, processes to produce a higher life form (if more than traditional breeding techniques), processes to produce an organ or a tissue through genetic transformation; processes for the in vitro culturing or manipulation of cells; processes to separate cells; and processes to generate mutants using a chemical or physical agent.
Example 1:
An application discloses a need for a new insect-resistant cotton plant. The description discloses a process for producing an insect-resistant transgenic plant, which requires the transformation of plant cells with a Bt toxin gene from a bacterium. Although transformation techniques were part of the common general knowledge of the person skilled in the art, it was not well known that insect resistance in cotton could be conferred by transforming plant cells with a Bt toxin gene.
Claims:
Analysis: The problem to be solved by the invention is determined to be a need to produce a new insect-resistant cotton plant. In this case, the solution is a process that relies on the transformation of a plant cell with a Bt toxin gene to generate a transgenic plant, which is followed by steps of traditional breeding that ultimately produce an insect resistant plant. Given that steps a) through e) provide the identified solution, these steps are all considered essential elements of claim 1. Thus, claim 1 defines statutory subject-matter since step a), which involves significant human invention, is an essential element of the claim. The fact that the process yields a non-statutory product (a plant) has no effect on the patentability of the process.
It should be emphasized that a proper assessment for patentability must be based on a purposive construction of the claim and not simply on a literal interpretation of the claim. For example, consider a different scenario in which it was common general knowledge that insect-resistant cotton plants are produced by transforming plant cells with a Bt toxin gene. In this scenario, the inventor discovered that the existing process, which uses transgenic Bt toxin plants, could be improved by using cotton variety B in crossbreeding. Thus, given that the person skilled in the art would recognize that transforming a cotton plant with a Bt toxin gene represents a commonly known solution to a commonly known problem, the problem would instead be viewed as a need to improve the process for producing insect-resistant cotton plants. In this case, the solution is based on the use of variety B in the breeding process, as represented by steps c) to e) of the claim. Given that the essential elements of the claim are limited to steps of traditional breeding [steps c) to e)], the claim would not define a statutory invention as defined in section 2 of the Patent Act.
Claim 2 is non-statutory. The subject-matter of the claim defines a higher life form and no degree of human intervention in its production can change the determination that it falls outside the definition of invention in section 2 of the Patent Act [see 23.02.01].
Example 2:
The description discloses a need for biological systems that can be used to screen cancer therapeutics. The description discloses a novel and inventive process for producing a skin-equivalent that is useful for screening potential anti-melanoma drugs.
Claims:
Analysis: Given that the process of claim 1 requires significant human intervention, and the end result of the process, the skin equivalent (claim 2), is functionally and anatomically distinct from natural skin [see 23.02.02], the subject-matter of these claims is not excluded from the scope of section 2 of the Patent Act. Therefore, the claims are statutory.
Example 3:
An application discloses a need for a sheep breed exhibiting the desirable trait of decreased wool fibre diameter and a need for an improved breeding method to produce such sheep. The inventors screened sheep for a genetic polymorphism and disclosed that a genetic marker (BAA81) on chromosome 11 correlated to the desired trait. Marker assisted selection was performed to identify sheep having the marker. In brief, DNA primers specific to the region surrounding the BAA81 marker were created, the primers were mixed with genomic DNA isolated from a sheep and PCR was performed. Sheep selected by this process were mated to produce progeny that exhibited significantly decreased fibre diameter compared to sheep lacking the marker.
Claim:
Analysis: It is clear that identification of the BAA81 region on chromosome 11 was not part of the common general knowledge. Recognizing that the problem to be solved was to produce sheep that have decreased wool fibre diameter, the inventors solved the problem using an improved breeding method that relied on marker assisted selection to identify the BAA81 polymorphism (step a) and selective breeding of only those sheep having the marker (steps b and c). Hence, in this case, all the steps of the claimed method are essential to solving the problem. The claim defines statutory subject-matter within the scope of section 2 of the Patent Act since the method relies on significant human intervention (step a) and is not construed as being limited to steps of traditional breeding.
Date modified: January 2009
Biomolecules are chemical compounds, and claims to nucleic acids, polypeptides, proteins and peptides are therefore directed to statutory matter. Certain biomolecules, further, express information through their primary structure (i.e. their sequence).
The three-dimensional structure of a biomolecule is often of importance in understanding its biological activity and behavior. A claim to a biomolecule, defining the molecule in terms of its atomic coordinates, is statutory. In contrast, a claim to the three-dimensional atomic coordinates that represent the shape of the biomolecule in space is not statutory. The coordinates themselves are simply information, which is non-statutory.
Note that the exclusion from patentability of information does not depend on whether or not the information has been recorded on a carrier, nor on the nature of the carrier.
A computer model of a biomolecule which relies on the structural information of the biomolecule is not patentable, since the model itself equates to a graphical presentation of the underlying information. This exclusion extends to include generic computer systems and/or programs that have merely been configured to generate the model.
Computer models of biomolecules can be used in, for example, in silico screening methods. The mere presence of a computer model of a biomolecule in a method does not of itself render the method unpatentable.
Examples:
Date modified: January 2009
A method which provides a practical therapeutic benefit to a subject, even if this is not its primary or intended purpose, is considered to be a method of medical treatment and is therefore not patentable.[306] By way of examples, surgical, medical, dental and physiotherapeutic methods of treatment are non-statutory matter.
To be considered a method of medical treatment, the method should cure, prevent or ameliorate an ailment or pathological condition, or treat a physical abnormality or deformity such as by physiotherapy or surgery. Certain natural conditions such as ageing, pregnancy, baldness and wrinkles are not considered to be pathological, and methods to treat such conditions are therefore not proscribed.
Methods that involve performing surgery on the human or animal body are excluded, whether the effect of the surgery is therapeutic or not. Methods that involve the excision of tissue, organ, or tumour samples from the body are considered to be forms of surgery, and are excluded regardless of their reproducibility. The removal of fluids from the body such as by needle or cannula is not of itself surgery.[307] A method to remove fluids may nevertheless be proscribed if it otherwise involves surgery, such as in the placement of a cannula or stent in the body,[308] or if it lacks utility, e.g. for not being reproducible.
Claims which do not involve a step of surgery or provide a practical therapeutic benefit do not form part of the method of surgery or medical treatment exclusion.[309] Thus, certain methods of diagnosing a disease or medical condition, whether practised in vitro or in vivo,[310] of treating an animal solely to derive an economic benefit,[311] or for achieving a cosmetic result may be patentable.
As mentioned in subsection 16.10.02, use claims are permitted but are scrutinized closely to ensure they do not equate to a medical or surgical method, for example by the inclusion of a medical or surgical step (see 17.03.02).
Similarly, a claim which recites a dosage regime, or a prescribed dosage amount, may be directed to a method of medical treatment since dosage regimes and prescribed dosage amounts fall within the purview of a medical professional.[312] However, dosage forms, pharmaceutical packages or kits, which may physically embody a dosage regime or prescribed dosage amount, are considered patentable subject matter.[313]
The removal of the medical aspect of a claim may render it acceptable. Inclusion of terms such as “cosmetic”, “diagnostic” or “non-medical” in a claim may be taken as disclaimers to medical methods provided the description contains adequate support for such terminology and provided the claim can reasonably be understood to be directed to a non-medical method the results of which cannot reasonably be said to produce a practical therapeutic effect.
Examples:
Analysis: non-statutory, since the method is self-evidently a method of medical treatment.
Analysis: statutory, since rodents are not susceptible to human papilloma virus and do not derive any therapeutic benefit from the administration of the peptide.
Analysis: statutory, since the animals do not obtain any therapeutic benefit from the method, and the method has clear industrial applicability.
Analysis: Statutory because, in this case, there is a distinction between the concentration of the radioisotope-labelled antibody which is used for diagnosis and that which would provide a therapeutic effect. The proviso “without medically treating said tumour” therefore qualifies the amount of antibody used and restricts it to non- therapeutic concentrations.[314]
Analysis: Statutory, since the method is clearly a diagnostic method and has been drafted in such a manner that any acts required to obtain the necessary sample of breast tissue do not form part of the claimed invention.
Analysis: non-statutory, since step (i) involves a step (a needle biopsy) which equates to surgery.
Analysis: statutory, since a method wherein a disease is induced in an otherwise healthy subject is not a method of medical treatment, even if the so-induced disease is subsequently treated.
This section focuses on the patentability of claims to kits and packages in the context of medical inventions.
A “package” is generally understood as one or more components that are contained within conventional packaging material, such as a box, paper or plastic wrapping, or the like. The person skilled in the art would understand that a package may contain a single component, a plurality of the same component, one or more different components, or any combination of these without limitation. Where appropriate, a package may be defined more particularly as, for example, a commercial package or a pharmaceutical package.
A “kit” is generally understood as a specific type of package that contains two or more components.
When a kit contains a composition, such as a unit dosage form, which is composed of two or more ingredients that are formulated together, that single formulated product is considered as one component in the kit. Thus, one unit dose would not reasonably be considered as two separate components in a kit. The skilled person would understand that there is a difference between a “composition” and a “kit”, based on the plain and ordinary meaning of those terms.
When a pharmaceutical composition comprising an active ingredient is a component in a medical kit, the following is a non-exhaustive list of examples of what the second component may be: an instrument for administration, e.g., an applicator, empty syringe or graduated cup; a separate formulating excipient, adjuvant or potentiator; a separate activating agent, reagent, or buffer; an antiseptic wipe; a test strip; a separate product comprising a second active ingredient; or instructions defining the use. See 23.03.03b below for a more detailed discussion of instructions.
Date modified: November 2017
The subject-matter of a claim must be defined distinctly and in explicit terms, in accordance with subsection 27(4) of the Patent Act, because the claims define the subject-matter of the monopoly. The scope of a claim must be clear and definite from the perspective of the person skilled in the art.
The terms “package” and “kit” are used interchangeably at times. In some cases this leads to a lack of clarity or creates avoidable ambiguity within a claim or set of claims, contrary to subsection 27(4) of the Patent Act.
A kit would be understood as a specific type of package comprising at least two components so, in order to comply with subsection 27(4) of the Patent Act, the term “kit” must be construed as having a minimum of two components. Where the term “kit” is construed as consisting of only one component, the claim to the kit would not comply with subsection 27(4) of the Patent Act. For instance, a subsection 27(4) defect would be identified where the application defines a kit as consisting of only one component. A subsection 27(4) defect may also be identified in cases where the application states that a kit is an embodiment of the invention but does not explicitly describe the components of said kit and the examiner construes the kit as having only one component. In contrast, no defect would be identified in cases where either the description or claim unambiguously defines the kit as containing at least two components or where the examiner construes the kit as containing at least two components.
If a package claim defines two or more components then there would be no lack of clarity even though the subject-matter could have been claimed as a kit. There are no restrictions on the number of components a package may contain.
A patent application may contain multiple independent product claims within the same claim set, such as claims to a package, a kit, and a package containing the kit, as long as the existence of the multiple product claims does not result in a lack of clarity.
Example
An application discloses that compound A, a known herbicide, has therapeutic utility for treating disease Y in humans. The description states that compositions comprising compound A may be formulated for a variety of routes of administration, but focuses on subcutaneous and intravenous injectable formulations and liquid oral formulations. In one embodiment the formulation and an empty syringe may be packaged together within a kit. The description also discloses using the formulation in combination with a second compound that also treats disease Y, and refers to a number of compounds well known for treating Y. Also described is an embodiment where compound A is packaged together with a second compound for treating disease Y.
Claims:
Analysis: Claim 1 complies with subsection 27(4) of the Patent Act. The claim is directed to a pharmaceutical composition comprising at least two ingredients, namely compound A and a pharmaceutically acceptable formulating excipient. The excipient is defined in broad terms but the nature and scope of the excipient would be clear to the skilled person based on their common general knowledge and in view of the specification as a whole, based on the terms “pharmaceutically acceptable” and “formulating”.
Claim 2 complies with subsection 27(4) of the Patent Act. The claim is directed to a kit comprising the pharmaceutical composition of claim 1. The claim only explicitly defines one component of the kit, namely the composition, and there are no indications in the claim relating to the nature of a second component. However, given that there is a basis in the description for what the second component of the kit may be, e.g. a syringe or the additional compound for treating disease Y, the scope of the claim would be understood as comprising at least two components and, therefore, satisfies subsection 27(4) of the Patent Act.
Claim 3 complies with subsection 27(4) of the Patent Act. The claim is directed to a kit comprising the pharmaceutical composition of claim 1 and an instrument for administering the composition. The instrument is defined in broad terms, but the nature and scope would be clear to the skilled person, based on their common general knowledge and in view of the specification as a whole. Notably, the claim would have also complied with subsection 27(4) if the second component of the kit was defined as the additional compound for treating disease Y.
Claim 4 complies with subsection 27(4) of the Patent Act. The claim is directed to a package comprising the kit of claim 2. As discussed above for claim 2, the kit satisfies subsection 27(4). In this case, the placement of the kit within a package does not lead to a lack of clarity and, therefore, the subject-matter of claim 4 also satisfies subsection 27(4) of the Patent Act. Notably, claim 4 would also be compliant with subsection 27(4) if it referred to claim 3 instead of claim 2.
Note that the claims must still be assessed for compliance with the other requirements of patentability.
Date modified: November 2017
Instructions are generally understood as information printed or displayed on a substrate. In the context of medical inventions, this information often suggests actions or directions that can be taken, such as how an active agent can be administered or used in treatment.
Instructions may be claimed as a secondary component of a kit or package; however, there is no general requirement that a kit or package comprise instructions.
Where a use is defined in the preamble or body of a claim or as part of the instructions, the claim may be construed as a “kit for use” or “package for use”, which is distinct from a claim to a kit or package per se. For instance, claims such as “a kit comprising A and B and instructions for using A and B to treat disease Y” and “a kit for treating disease Y comprising A and B” are both construed as “kit for use” claims.
Example
An application discloses there is a need for improved treatment of painful diabetic neuropathy in patients. The description states that the inventors have surprisingly discovered that levetiracetam and carbamazepine (known anti-epileptic drugs), when used in combination, are effective for reducing pain associated with diabetic neuropathy.
Claims:
A search of the prior art identified patent document D1, which discloses the combined use of levetiracetam and carbamazepine in epileptic patients. An embodiment of D1 includes a kit comprising both levetiracetam and carbamazepine as well as instructions for preventing seizures in patients.
Analysis: Claims 1 and 2 comply with subsection 27(4) of the Patent Act. Claim 1 recites a kit comprising a first pharmaceutical formulation comprising levetiracetam and a second pharmaceutical formulation comprising carbamazepine. The scope of claim 1 would be understood as a kit containing at least two components, namely the first and second pharmaceutical formulations. In claim 2, the skilled person would understand that the instructions, which define the use of levetiracetam and carbamazepine, represent an additional component of the kit. In view of this, the claims satisfy subsection 27(4) of the Patent Act.
In regard to the requirement for novelty, claim 1 is anticipated by D1 because D1 discloses and enables a kit comprising both levetiracetam and carbamazepine. Recognizing that the kit of claim 2 further comprises instructions for using levetiracetam and carbamazepine to treat pain associated with diabetic neuropathy and that D1 does not disclose and enable this use, claim 2 is novel over D1. Thus claim 2 is regarded as a new use of a kit comprising levetiracetam and carbamazepine that complies with section 28.2 of the Patent Act.
Note that the claims must still be assessed for compliance with the other requirements of patentability.
Date modified: November 2017
The examination of patent applications featuring medical diagnostic method claims presents certain challenges and warrants specific guidance to ensure efficient, predictable, and reproducible examination.
A diagnostic method outlines a sequence of steps to be followed to extract diagnostic meaning from data and will often comprise steps to:
In order to determine the patentability of a diagnostic method claim, the examiner must take into account the general guidance on purposive construction in Chapter 12 of this manual, which involves a determination of the problem addressed by the application, the solution as contemplated by the inventor and the essential elements that provide the solution. It follows that an evaluation for compliance with section 2 of the Patent Act is to be made on the basis of the essential elements as determined through a purposive construction.
The guidance herein may be applicable to claims in a form such as:
Date modified: November 2017
The identification of the problem and the solution provided by the invention informs the purposive construction of the claims.[316] An identification of the problem is guided by the description and the examiner's understanding of the common general knowledge in the relevant art.
Examiners should bear in mind that an application may describe more than one problem to be solved. For diagnostic methods, it may be appropriate to consider that an inventor is generally looking to solve a data acquisition problem and/or a data analysis problem.
Where a data acquisition problem exists, the description will typically describe technical matter that goes beyond the common general knowledge (CGK) of the skilled person in the art. Factors in the description that may indicate the existence of a data acquisition problem include:
Factors in the description that may suggest that a data analysis problem exists include:
Once the problem is identified, the examiner must determine the solution to the problem as contemplated by the inventor. In some cases, the problem may not be readily apparent and an identification of the solution may actually inform the problem addressed by the invention.
Date modified: November 2017
Recall from Chapter 12 that the solution is the element or set of elements that is essential to the successful resolution of the problem. If a claim includes solutions to more than one problem, examination should focus on one solution to a problem in performing the purposive construction. The initial choice of solution should be guided by the description, selecting the solution given the greatest emphasis by the inventors. If it becomes necessary to consider a different solution, the analysis should be undertaken anew.
Where a data acquisition problem has been identified, the solution is provided by those elements that provide a means to acquire data about an analyte. The means by which the data is acquired may be represented by either a single step or by multiple steps within the diagnostic claim.
For example, elements relating to data acquisition may be represented by steps such as:
Where a data analysis problem has been identified, the solution is provided by those elements that relate to the analysis of acquired data for the purpose of providing diagnostic meaning.
For example, elements relating to data analysis may be represented by steps such as:
Date modified: November 2017
Having identified the problem and solution, a purposive construction of the claims involves:
Recognizing that how data is analyzed or interpreted in a diagnostic method generally has no material effect on how the data needs to be physically acquired (and vice versa), the data acquisition elements and data analysis elements in the diagnostic method claim likely have a relationship reflecting an aggregation rather than a combination. Thus, the solution to a problem will be provided by either data acquisition elements or data analysis elements, but not both.
Where a data acquisition problem exists, the essential element or set of essential elements providing the solution is the means to acquire data about an analyte. If the identified problem does not relate to data acquisition then it will presumably relate instead to a data analysis problem. Where this is the case, the essential elements will include steps relating to the mental analysis and/or intellectual significance of the data and will likely not include any steps to acquire the data since the way the data is acquired does not change the nature of the solution (e.g., how X is detected or measured in a sample will not change the intellectual significance of its presence).
Date modified: November 2017
A diagnostic claim construed as being limited to essential elements that are disembodied (e.g., mental process, lacking physicality, no practical application, etc.) will be identified as defective for not complying with section 2 of the Patent Act because the subject-matter does not fall within a category of invention as defined in section 2. This would generally apply to situations where the identified solution is only provided by an element or set of elements associated with the analysis or significance of the acquired data (e.g., correlation of a marker to a disease).
By contrast, data acquisition elements likely define statutory subject-matter since they usually relate to tangible (non-disembodied) practical steps which fall within a category of invention as defined in section 2. Thus, where such a data acquisition element is identified as an essential element of the construed claim, the claimed subject-matter will likely be statutory unless the claim includes excluded subject-matter, such as a method of medical treatment.
Date modified: November 2017
Example 1:
The following background information is applicable to all scenarios within Example 1. Each scenario will provide separate additional information about the prior art and/or CGK.
The specification describes a method of diagnosing whether a patient is at risk for developing thyroid cancer.
Claim:
Scenario 1A:
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is a team including an oncologist, an endocrinologist, a geneticist, a molecular biologist and a medical technologist.
Common general knowledge (CGK)
The CGK of the POSITA included knowledge of cancer treatment and diagnosis as well as conventional genotyping techniques. At the claim date, gene XYZ was a well-known signaling pathway gene and the gene, as well as data available in public databases about the gene, were CGK to the POSITA. In this scenario, mutation A in gene XYZ was not CGK to the POSITA.
The Problem
It is clear from the description and CGK that there is more than one problem to be solved by this invention. Given that the application discloses a need to identify a biomarker that correlates to thyroid cancer risk, this is suggestive that a data analysis problem exists. The description also makes apparent that the inventors are proposing a solution to a data acquisition problem since a mutation at position 123 of gene XYZ was not CGK to the POSITA and, by extension, methods of detecting and specifically acquiring data about the nucleotide at position 123 were also not CGK. Recognizing that means for specifically detecting the nucleotide at position 123 of gene XYZ were not CGK and that the description details how this is detected, a purposive construction will be based on the data acquisition problem: a need to detect and identify the nucleotide at position 123 of gene XYZ in a human subject.
The Solution
The identified data acquisition problem is solved by the provision of a method that, when practised:
What are the essential elements?
As the solution to the data acquisition problem is provided by steps (a) and (b) of the claimed method, these steps are essential elements of claim 1.
Statutory subject-matter – section 2
Claim 1, as construed, is statutory because the essential elements of the claim define subject-matter that falls within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
Although the full nucleotide sequence of gene XYZ was known in the prior art, the prior art did not specifically disclose means to detect the nucleotide at position 123 in known gene XYZ in a biological sample from a human subject and specifically acquire data about the identity of the nucleotide at position 123. Therefore, the claim is novel because there is no single prior art disclosure that discloses and enables the essential elements of the claim.
Obviousness – section 28.3
Based on a reading of the specification as a whole from the perspective of the POSITA, in light of their CGK, the inventive concept of the claim includes a method that provides both a means for detecting the identity of the nucleotide at position 123 of gene XYZ within a biological sample, and the specific acquisition of data about the identity of the nucleotide at that position. Considering the prior art, it is apparent that genotyping techniques were well known at the claim date and the full length nucleotide sequence of gene XYZ (including position 123) was available to the POSITA from public databases. However, the difference between the prior art and the inventive concept is that the prior art did not disclose looking specifically at position 123 of gene XYZ in order to acquire data about the identity of the nucleotide at that position. The difference does not constitute a step that would have been obvious to the POSITA. Therefore, the construed claim is inventive.
Regarding claim 1 in scenario 1A:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
Y |
As the claim meets all of the requirements of patentability the claim is allowable.
Scenario 1B:
D1 discloses that nucleotide position 123 of gene XYZ has been determined to be a mutational hotspot across a population of tumour samples. Methods used to specifically identify a mutation at this position are also described. This information was not CGK to the POSITA.
Analysis:
Since neither the description nor the CGK have changed relative to Scenario 1A, the POSITA, CGK, problem, solution and essential elements remain as they were stated in that scenario. The analysis below takes into consideration the disclosure of prior art document D1.
Statutory subject-matter – section 2
Claim 1, as construed, is statutory because the essential elements of the claim define subject-matter that falls within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
The claim lacks novelty in view of D1 because D1 discloses and enables the essential elements of claim 1, namely means for the identification of the nucleotide at position 123 of gene XYZ in a biological sample and the acquisition of specific data about the identity of the nucleotide at that position. It should be noted that, in this case, the actual identity of the nucleotide at position 123 (e.g., whether it is A, T, C or G) is not part of the claim or the essential elements.
Further, although D1 did not disclose that a mutation at said position correlates to thyroid cancer risk, the claim is anticipated because this correlation is not an essential element of the data acquisition problem.
Obviousness – section 28.3
The claim is obvious in view of D1 because it was already determined that the claim is anticipated by D1 (see MOPOP 18.02.02d). For the sake of completeness, the examiner determines that the inventive concept of the claim includes a method that provides both a means for identifying the nucleotide at position 123 of gene XYZ within a biological sample, and the specific acquisition of data about the identity of the nucleotide at that position. D1 discloses means for identifying the nucleotide at position 123 of gene XYZ in a biological sample and acquiring specific data about the identity of the nucleotide at that position. It is evident that there is no difference between the inventive concept of the claim and D1 and, therefore, the POSITA would not have required any degree of invention to arrive at the inventive concept.
It should be noted that the data analysis elements do not form part of the inventive concept as the examiner has determined that a data acquisition problem was solved.
Regarding claim 1 in scenario 1B:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
N |
|
Non-obvious 28.3 |
N |
The claim is not allowable as it does not meet all of the requirements of patentability
Scenario 1C:
Each of D2-D8 independently discloses testing human subjects for prostate cancer by determining the identity of the nucleotide at position 123 and looking at whether mutation A exists at that position. The examiner has determined that both the means for determining the identity of the nucleotide at position 123 in a biological sample from a human subject and the link between mutation A and prostate cancer were CGK.
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is a team including an oncologist, an endocrinologist, a geneticist, a molecular biologist and a medical technologist.
Common general knowledge (CGK)
The CGK of the POSITA included knowledge of cancer treatment and diagnosis as well as conventional genotyping techniques. At the claim date, gene XYZ was a well known signaling pathway gene and the gene, as well as data available in public databases about the gene, were CGK to the POSITA. In this scenario, both mutation A in gene XYZ and the means of determining whether this mutation was present in gene XYZ at nucleotide position 123 in a sample were CGK to the POSITA (see D2-D8). Further, the link between mutation A at position 123 and prostate cancer was CGK.
The Problem
Considering the specification as a whole and the background of the CGK in the relevant field, the examiner has determined that a problem related to data analysis exists. More particularly, the problem appears to be related to a need to correlate a particular genotype in a human subject with a risk of developing thyroid cancer. Although the specification also describes methods for acquiring data about the mutation at nucleotide position 123 of gene XYZ, it is apparent that the inventors are not proposing a solution to a data acquisition problem of how to determine the sequence at position 123 of gene XYZ because its solution already existed in the CGK (see D2-D8).
The Solution
The solution to the identified data analysis problem was arrived at by the discovery of a correlation between the presence of a mutation at position 123 of gene XYZ and thyroid cancer.
What are the essential elements?
As the solution to the data analysis problem is represented by step (c) of the claimed method, the essential element of the claim relates to the correlation between mutation A at position 123 and the risk of thyroid cancer.
Statutory subject-matter – section 2
Claim 1, as construed, is not statutory because the essential element of the claim defines subject-matter that is disembodied and does not fall within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
The claim is novel because there is no single prior art disclosure that discloses and enables the essential element of the claim, namely the correlation between mutation A at position 123 and the risk of thyroid cancer.
It should be noted that although each of D2-D8 independently discloses and enables a method for identifying the nucleotide at position 123 of gene XYZ in a biological sample, the claim is not anticipated by any of D2-D8 because the data acquisition steps in the claim that correspond to the means of detection are not essential elements of the data analysis problem.
Obviousness – section 28.3
Based on a reading of the specification as a whole from the perspective of the POSITA, in light of their CGK, the inventive concept of the claim is the correlation between the presence of mutation A at position 123 of gene XYZ and thyroid cancer. Taking into consideration the information disclosed in D2-D8 and the CGK, it is apparent that mutation A at position 123 of gene XYZ was associated with prostate cancer. However, the prior art does not disclose an association with thyroid cancer. The examiner has concluded, in this case, that the POSITA would not have considered the association between mutation A at position 123 of gene XYZ and thyroid cancer to have been obvious at the claim date. Therefore, the construed claim is inventive.
Regarding claim 1 in scenario 1C:
|
Statutory subject-matter s2 |
N |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
Y |
The claim is not allowable as it does not meet all of the requirements of patentability.
It should be noted that if the applicant argues that a data acquisition problem (not a data analysis problem) was solved by their invention, the examiner would provide an alternative data acquisition problem analysis in a subsequent report.
Example 2:
The specification describes an improved method for diagnosing disease P, which is a lysosomal storage disease.
Claim:
Analysis:
Person of ordinary skill in the art (POSITA)
The POSITA is a team including a medical practitioner, a biochemist, and a medical technologist.
Common general knowledge (CGK)
The CGK of the POSITA included knowledge of disease P and other lysosomal storage diseases, as well as existing biochemical assays for diagnosing such diseases. In this example, it was CGK to diagnose disease P by carrying out enzyme assays on skin samples. It was not CGK to measure enzyme E activity in blood samples.
The Problem
Considering the specification as a whole and the background of the CGK of the POSITA in the relevant field, the examiner has determined that the problem relates to data acquisition. Specifically, the identified problem is a need for an improved method of measuring enzyme E activity in a biological sample from a human subject. This conclusion was based on the fact that the instant description details an improved assay method dependent on sample selection which represents a solution that did not exist in the CGK prior to the invention.
The Solution
The identified data acquisition problem is solved by the provision of an improved method that, when practised:
What are the essential elements?
As the solution to the data acquisition problem is provided by steps (a) and (b) of the claimed method, these steps are essential elements of claim 1.
Statutory subject-matter – section 2
Claim 1, as construed, is statutory because the essential elements of the claim define subject-matter that falls within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
The claim is novel because there is no single prior art disclosure that discloses and enables the essential elements of the claim.
Obviousness – section 28.3
Based on a reading of the specification as a whole from the perspective of the POSITA, in light of their CGK, the inventive concept of the claim includes an improved method which includes steps for measuring enzyme E activity by carrying out the measurement by mass spectrometry on a sample of dried blood and specifically acquiring data about the activity of enzyme E. With respect to the prior art, D1 is considered the closest prior art and discloses a method of measuring enzyme E activity. The method of claim 1 differs from D1 in that D1 discloses that the enzyme assay was carried out on cultured skin cells while the instant method uses dried blood samples. This difference, however, does not amount to an inventive step in view of D2. The POSITA would have come directly and without difficulty to measure enzyme E activity in dried blood samples using mass spectrometry given that D2 disclosed that the use of such samples exhibited an advantage over the use of cultured skin cells in assays for other enzymes implicated in related lysosomal storage diseases. Therefore, the claim is obvious in view of a D1 when combined with D2.
Regarding claim 1 in Example 2:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
N |
The claim is not allowable as it does not meet all of the requirements of patentability.
Example 3:
The specification describes a method of diagnosing gastrointestinal infections based on the presence of combinations of markers in stool samples.
Claim:
Person of ordinary skill in the art (POSITA)
The POSITA is a team including a medical practitioner, a microbiologist, and a medical technologist.
Common general knowledge (CGK)
The CGK of the POSITA included knowledge of gastrointestinal infections and associated pathogenic bacteria. Means for detecting two or more of markers selected from G, U, T and S together in a stool sample were not CGK to the POSITA.
The Problem
Considering the specification as a whole and the background of the CGK of the POSITA in the relevant field, the examiner has determined that the problem relates to data acquisition. Specifically, the identified problem relates to the detection of combinations of two or more of markers G, U, T and S in a sample.
The Solution
The identified data acquisition problem is solved by the provision of a method that provides means for detecting combinations of two or more markers selected from G, U, T and S within the same stool sample; and that specifically acquires data about the presence of these markers.
What are the essential elements?
As the solution to the data acquisition problem is provided by steps (a) and (b) of the claimed method, these steps are essential elements of claim 1.
Statutory subject-matter – section 2
Claim 1, as construed, is statutory because the essential elements of the claim define subject-matter that falls within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
The claim is novel. D1 does not anticipate the construed claim because it does not disclose the detection of a combination of two or more of markers within the same stool sample.
Obviousness – section 28.3
Based on a reading of the specification as a whole from the perspective of the POSITA, in light of their CGK, the inventive concept of the claim is a method that provides means for detecting combinations of two or more markers selected from G, U, T and S within the same stool sample and specifically acquiring data about their presence in the sample. D1 represents the closest prior art and discloses the specific association of each of markers G and U with bacterial strain X1, as well as methods for separately detecting each of the two markers in stool samples. D1 does not disclose that the methods provide steps for detecting the combination of the two markers in the same sample. However, this difference does not constitute an inventive step. In view of D1, the POSITA would have been aware that both proteins G and U act as markers for the same strain and the POSITA would have come directly and without difficulty to the method of detecting the combination of both G and U within the same stool sample. Therefore, the examiner determines that, in this case, the claim is obvious in view of D1 and the CGK of the POSITA.
Regarding claim 1 in Example 3:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
N |
The claim is not allowable as it does not meet all of the requirements of patentability.
Example 4:
The specification describes a method for determining the risk of developing diabetes associated with exposure to persistent organic pollutants (POPs).
Claims:
Purposive construction for claims 1 and 2:
A purposive construction analysis is set out below for claims 1 and 2 because these claims include both data acquisition and data analysis elements related to medical diagnoses.
As the examiner has determined that claim 3 is not a diagnostic method and defines statutory subject-matter, a purposive construction analysis has not been set out for claim 3. Only the examiner’s conclusions as to novelty and inventiveness are provided below for claim 3.
Person of ordinary skill in the art (POSITA)
The POSITA is a team including a medical practitioner, a toxicologist, an endocrinologist and a medical technologist. Further, the POSITA is skilled in gene expression analysis using microarrays.
Common general knowledge (CGK)
The CGK of the POSITA included knowledge of POPs and the health effects associated with POP exposure and bioaccumulation, as well as knowledge of insulin-related metabolic diseases. The link between POP exposure and diabetes was also CGK but the CGK did not include any knowledge of associated genetic markers. The CGK also included the use of commercial microarrays to simultaneously measure the expression levels of a plurality of genes. As admitted in the description, each of genes T, O, X, I and C were represented, amongst thousands of other genes, on a single commercial microarray. It was not CGK, however, to both 1) specifically measure the expression levels of genes T, O, X, I, and C and 2) specifically acquire the data about the expression levels of T, O, X, I and C (while disregarding the levels of all other genes).
The Problem
Considering the specification as a whole and the background of the CGK of the POSITA in the relevant field, the examiner has determined that a problem the inventors set out to address relates to data acquisition. Specifically, the identified problem relates to the determination of the expression levels of only genes T, O, X, I and C in a patient’s sample.
The Solution
The identified data acquisition problem is solved by the provision of a method that both 1) specifically measures the expression levels of genes T, O, X, I and C, and 2) specifically acquires data about the expression levels of only these genes.
What are the essential elements?
As the solution to the data acquisition problem is provided by step (a) of claim 1, this step is an essential element of claim 1.
In claim 2, elements of the claim that provide means to acquire data about the expression levels of genes T, O, X, I and C are essential because they give the solution to the identified data acquisition problem. However, the use of the microarray to determine the risk of developing POP-associated diabetes is not an essential element of claim 2 because it provides the solution to a data analysis problem.
Statutory subject-matter – section 2
Claims 1 and 2, as construed, are statutory because the essential elements of the claims define subject-matter that falls within a category of invention as defined in section 2 of the Act.
Novelty – subsection 28.2(1)
Claims 1 and 2 are novel. The prior art does not anticipate the construed claim because no single document discloses the essential element of the claims. Although D1 discloses a microarray that is capable of measuring the expression levels of thousands of genes, including T, O, X, I and C, D1 does not anticipate claim 1 or 2 because the data set acquired from D1 is not specific to data about the expression levels of genes T, O, X, I and C alone and D1 does not teach looking specifically at these particular genes.
Claim 3 lacks novelty in view of D1, which discloses and enables a microarray comprising oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C and wherein each probe is attached to a solid support at a discrete position.
It should be noted that if the term “comprising” in claim 3 was replaced by the term “consisting”, claim 3 would be novel if a microarray consisting solely of oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C was not disclosed in the prior art.
Obviousness – section 28.3
Based on a reading of the specification as a whole from the perspective of the POSITA, in light of their CGK, the inventive concept of claims 1 and 2 is specifically measuring the expression levels of genes T, O, X, I and C in a patient’s sample using a microarray comprising oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C and wherein each probe is attached to a solid support at a discrete position, and specifically acquiring data about the expression levels of these genes. Considering the prior art, it is apparent that the use of commercial microarrays to simultaneously measure the expression levels of a plurality of genes was well known at the claim date. Further, microarrays comprising oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C were known from D1. However, the difference between the prior art and the inventive concept is that the prior art did not disclose that expression level data about only T, O, X, I and C was specifically acquired and D1 did not disregard data about the expression levels of the remaining 22,000 genes on the array. The difference does not constitute a step that would have been obvious to the POSITA. Therefore, claims 1 and 2 are inventive.
Claim 3 is obvious in view of D1 because it was already determined that the claim was anticipated by D1 (see MOPOP 18.02.02d). For the sake of completeness, the examiner determines that the inventive concept of claim 3 is a microarray comprising oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C and wherein each probe is attached to a solid support at a discrete position. Prior art document D1 also discloses and enables a microarray comprising oligonucleotide capture probes that are complementary to nucleic acids corresponding to T, O, X, I and C and wherein each probe is attached to a solid support at a discrete position. It is evident that there is no difference between the inventive concept of claim 3 and D1 and, therefore, the POSITA would not have required any degree of invention to arrive at the inventive concept.
Regarding claim 1 in Example 4:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
Y |
Regarding claim 2 in Example 4:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
Y |
|
Non-obvious 28.3 |
Y |
Regarding claim 3 in Example 4:
|
Statutory subject-matter s2 |
Y |
|
Novel 28.2(1) |
N |
|
Non-obvious 28.3 |
N |
The application is not allowable as claims 2 and 3 do not meet all of the requirements of patentability.
Date modified: January 2009
Closely related to the question of utility is that of sufficiency. Subsection 27(3) of the Patent Act requires (inter alia) that the description “correctly and fully describe the invention and its operation or use as contemplated by the inventor”. Thorson P. summarized the requirements for sufficient specification in Minerals Separation North American Corp v Noranda Mines, Ltd, and later described this “onus of disclosure” as “a heavy and exacting one”.[319]
The description must be correct; this means that it must be both clear and accurate. It must be free from avoidable obscurity or ambiguity and must be as simple and distinct as the difficulty of description permits. It must not contain erroneous or misleading statements calculated to deceive or mislead the persons to whom the specification is addressed and render it difficult for them without trial and experiment to comprehend in what manner the invention is to be performed. It must not, for example, direct the use of alternative methods of putting it into effect if only one is practicable, even if persons skilled in the art would be likely to choose the practicable method. The description of the invention must also be full; this means that its ambit must be defined, for nothing that has not been described may be validly claimed.[320]
As was noted in section 19.01.03c, the description must contain sufficient information to support a sound prediction of the utility of the invention. Further, it must set out the invention such that a person skilled in the art can practice it having reference only to the description itself and to common general knowledge.
In Consolboard, Dickson J. noted that “the inventor must, in return for the grant of a patent, give to the public an adequate description of the invention with sufficiently complete and accurate details as will enable a workman, skilled in the art to which the invention relates, to construct or use that invention when the period of the monopoly has expired”
.[321] The description must be able to answer the questions “What is your invention?: How does it work?”[322] such that “when the period of monopoly has expired the public will be able, having only the specification, to make the same successful use of the invention as the inventor could at the time of his application”
.[323]
A description sufficient to allow the public (in the form of a person skilled in the art) to practice the invention is said to be enabling. Since the person skilled in the art is the addressee of the description, it is not necessary for common knowledge to be comprehensively disclosed. A known assay technique does not need, for example, to be taught in full. Merely referring to this technique is sufficient for the person skilled in the art to know how to practise it.
When an examiner has reason to believe that a description is deficient for not having correctly and fully described the claimed invention, an objection is raised under subsection 27(3). This might be the case, for example, when a broad claim is supported only by its own verbatim language.
It is important to bear in mind that the specification must be sufficient to allow the full scope of the claimed invention to be practised without the need for the person skilled in the art to exercise their inventive ingenuity. If the person skilled in the art is called on to solve problems in such a manner that an inventive step would be present, the description is insufficient (and the attendant claims are unsupported).
Date modified: March 2016
The following subsections relate to issues regarding nucleic acids, polynucleotides, peptides, polypeptides and proteins and the disclosure of their sequences in a sequence listing.
Date modified: July 2022
Generally a product may be defined by its structure, in terms of the process by which it is made, or in terms of its physical or chemical properties. Often the most explicit and definite manner in which to define a chemical compound is by its structure.
For a biomolecule such as a nucleic acid molecule or protein, the structure is typically represented by the nucleotide or amino acid sequence, e.g., “a polypeptide consisting of the amino acid sequence MARNDCQEGHILKFPSTWYV as set forth in SEQ ID NO:2”.
For greater clarity, the claim should be explicitly directed to a biomolecule defined by reference to a sequence identification number that points to the corresponding sequence in the sequence listing [see 23.05.07], e.g., “a nucleic acid consisting of the nucleotide sequence represented by SEQ ID NO:1” or “a protein comprising the amino acid sequence set forth in SEQ ID NO:2”. A claim simply directed to a sequence identification number, however, may be interpreted as a claim to mere information (i.e., to the string of letters depicted in the sequence listing), which is not compliant with section 2 of the Patent Act, rather than a claim to the biomolecule itself. A claim directed to “SEQ ID NO:8”, for example, would be unacceptable but a claim to “DNA encoding the protein comprising amino acids 1-260 of SEQ ID NO: 8” would be unambiguous (assuming the reference sequence is clearly defined – see below).
Note that even where a claim to a biomolecule is defined by reference to a sequence within the sequence listing it is not an assurance that the claimed biomolecule will be adequately defined by structure. For example, where a biomolecule is defined in a claim by reference to a sequence that contains a number of variable symbols, such as “X” or “n”, the claimed subject-matter may not be defined in distinct and explicit terms and may fail to comply with subsection 27(4) of the Patent Act.
In the case of a nucleic acid molecule defined by the protein it encodes, the provision of a partial amino acid sequence of the protein is not taken as an adequate description of a nucleic acid molecule which is capable of encoding the entire protein.[324]
Date modified: October 2019
Functional language is generally used to provide breadth to a claim. In certain cases, language that defines specific functional or biological activity may be used to further distinguish a claimed biomolecule from biomolecules of the prior art. Although the use of functional language does not make a claim defective per se, if it is used then the entire scope of the claim must be clear and fully supported by the description [see Chapter 14 of this manual for more information].
In general, the use of functional language in a claim is acceptable if the person skilled in the art would not need to resort to inventive ingenuity to practise the full scope of the claim. For example, consider a claim to “a plant transformation vector comprising a gene of interest; a transposon; and a marker gene positioned within the transposon, wherein the marker gene induces abnormal cellular differentiation in plant tissue”
.
Assuming that representative marker genes are adequately supported in the description and are well known in the prior art by persons skilled in the art, it is acceptable in this case to define the marker gene in functional terms.
On the other hand, where the use of functional language requires the person skilled in the art to exert an inventive effort to practise the full scope of the claim or, likewise, the use of the language causes the scope of the claim to be overly-broad, the claim is likely defective in view of section 60 of the Patent Rules. Where the examiner determines that the description is insufficient to support the breadth of the claim, depending on the facts, a defect could be identified under subsection 27(3) of the Patent Act. Where knowledge of the structure of the protein or nucleic acid is needed to realize the full scope of the claim, the claim may also lack compliance with subsection 27(4) of the Patent Act if the nucleic acid or protein is not further defined by the structure that provides the functional activity.
In the case where, for example, the structure of a protein (or a nucleic acid encoding a protein) is defined in terms of a percent identity to a reference sequence, the claim should additionally specify that the protein has the same biological activity as that described in the application in order to comply with subsection 27(4) of the Patent Act– e.g., “a nucleic acid comprising a sequence that is at least 90% identical to SEQ ID NO:1 which encodes a protein having alpha-amylase activity”.
Example:
An application describes a novel polypeptide depicted in the sequence listing as SEQ ID NO:2 that has xylanase activity and is shown to be particularly effective in processes for making biofuels. The description does not describe any variants of the polypeptide having xylanase activity. A search of the prior art revealed that xylanases are generally known. A search for the amino acid sequence of SEQ ID NO:2 identified prior art documents D1 and D2. D1 discloses a polypeptide having 82% sequence identity to SEQ ID NO:2 but lacking xylanase activity while D2 discloses a xylanase having 92% sequence identity with SEQ ID NO:2.
Claims:
Analysis: claim 1 is defective. The claimed polypeptide is defined broadly by a functional description of its activity rather than by its structural features. The description discloses with particularity only one polypeptide; this polypeptide is described as having the structural features depicted in SEQ ID NO:2 and the desired xylanase activity. Given that the claim defines more than the description supports, the claim is defective in view of section 60 of the Patent Rules. Where the examiner determines that the description is insufficient to support the breadth of the claim, depending on the facts a defect could be identified under subsection 27(3) of the Patent Act. The subject-matter of the claim also lacks novelty in view of D2 (in effect, the claim would be anticipated by any earlier public disclosure of a polypeptide having the desired activity). D1, on the other hand, would not be anticipatory to claim 1 since D1 does not disclose a polypeptide having the specified activity. Furthermore, if, having regard to the claim and description, it is not clear to the skilled person what is being claimed then a defect under subsection 27(4) of the Patent Act may also be identified.
Claim 2 is defective on multiple grounds. The polypeptide is defined in terms of its structure and, more particularly, to the minimum threshold of percent identity of the structure to the amino acid sequence of SEQ ID NO:2. In this case the claim defines more than the description supports and does not comply with section 60 of the Rules. Given that claim 2 does not define the functional activity of the polypeptide, the claim potentially encompasses polypeptides that lack xylanase activity and/or have unknown function. Identification of a defect under subsection 27(4) of the Patent Act may be warranted where it is unclear whether what is being claimed has the same functional activity as the polypeptide of the application. In addition, the claim is defective for lacking novelty in view of either D1 or D2, which each disclose and enable a polypeptide comprising an amino acid sequence that is “at least 80% identical to SEQ ID NO:2”. Had claim 2 included a functional limitation to xylanase activity then D1 would not have been anticipatory.
Date modified: March 2016
Nucleotide or amino acid sequences referred to as being “substantially identical” to a target sequence are not adequately defined since there is no accepted convention in the art as to what is encompassed by the term “substantially” and since the scope of a claim may vary depending on what one considers to be a “substantially” identical sequence.
A nucleotide or amino acid sequence may be defined by a threshold percentage limit as compared to a target sequence – e.g., a nucleic acid molecule comprising a nucleotide sequence that is at least 95% identical to the sequence of SEQ ID NO: 7. If the term “homology” is used to describe the relationship between the sequence and the target then the claim is considered indefinite since the term implies an evolutionary relationship which either exists or does not exist.[325] Applicants are generally permitted to replace the term “homology” with the term “identity” for greater clarity. A defect under subsection 27(4) of the Patent Act may also be identified where a claim includes the term “similarity” and there is no clear definition of what the applicant considers to be similar residues.
Date modified: March 2016
Nucleic acids are often defined as sequences that hybridize to a particular target sequence under various reaction, or stringency, conditions. Given that there is no clear consensus as to what conditions are best used in a given hybridization reaction and that different reaction conditions will capture different nucleic acids, a claim may be held to be indefinite for failing to define the particular parameters to be used during the hybridization reaction and ensuing washings.
Where the target itself is solely defined as being any member of a large family of nucleic acids, e.g., a family of degenerate nucleic acids or variants encoding the same amino acid sequence (including nucleic acids defined as having less than 100% identity), the scope of a claim to a nucleic acid molecule that hybridizes to such a target becomes unclear. In such cases, the target is not limited to a single clearly-defined nucleic acid but instead encompasses a vast number of possible combinations of hybridizing and target nucleic acids.
Where a claim suggests that a nucleic acid molecule, which hybridizes to a target sequence encoding a functional polypeptide, is itself also capable of encoding a functional polypeptide, the claim may be held to be defective under subsection 27(4) of the Patent Act since hybridizing nucleic acids may either not encode a polypeptide, or encode a polypeptide having a different function than that encoded by the target. For greater clarity, such claims should indicate that the nucleic acid molecule hybridizes to the complement of the target sequence.
Date modified: March 2016
Whenever a sequence is identified as having a certain percent identity to a reference sequence, it is necessary to define in the claim whether the percent identity is relative to the full length of the reference sequence or is a partial alignment (such as a BLAST alignment[326]).
For the sake of clarity, alignment of the sequence over the full length of the reference sequence is greatly preferred when making the comparison.
Date modified: March 2016
In accordance with section 28.3 of the Patent Act, an invention as claimed cannot be obvious or, equivalently, must be the result of ingenuity[327] [see Chapter 18 of this manual for further guidance].
If given the amino acid sequence of a polypeptide, the entire class of nucleic acids encoding it can be generated through simple deduction; i.e., by using the genetic code to back-translate from the amino acid sequence. Therefore, where protein X is known in the prior art, a broad claim to “a nucleic acid encoding the amino acid sequence of protein X”, for example, is considered obvious.
The opposite is also considered obvious. An amino acid sequence encoded by a known nucleic acid can be directly derived through the translation of the known coding nucleotide sequence provided the correct reading frame has been identified or is obvious.
Given that the class of nucleic acids encoding any particular polypeptide is astronomically large, the identification of a species of the class which has unexpected or advantageous properties can be inventive. Such claims should be analyzed in the context of a selection [see Chapter 18 of this manual].
Example:
An application discloses that a nucleic acid molecule (SEQ ID NO:7) is particularly advantageous for expression in plant tissue and encodes a peptide having the amino acid sequence set forth in SEQ ID NO:8. Prior art document D1 discloses the amino acid sequence of peptide G, which is identical to SEQ ID NO:8, but was derived through Edman degradation. There are no indications in D1 that recombinant techniques were used nor is there an explicit disclosure of a nucleic acid molecule which encodes peptide G. Review article D2 discusses methods and codon usage tables that may be used in order to achieve enhanced expression of heterologous genes in plant tissues.
Claims:
Analysis: Although it is recognized that obviousness inquiries should follow a four‑step approach,[328] the analysis has been simplified for the purposes herein.
Claim 1 is obvious in view of D1. Firstly, the claim does not refer to any nucleic acid in particular and merely reflects the general idea of having a nucleic acid molecule which is capable of encoding the peptide; an idea that a person of skill in the art would readily appreciate in view of D1. Second, D1 provides the amino acid sequence of the peptide making it a simple matter of deduction for the person of skill in the art to generate a nucleotide sequence capable of encoding the peptide. Therefore, claim 1 fails to satisfy section 28.3 of the Patent Act in view of the teachings of D1.
Claim 2 is obvious in view of D1 in combination with D2. The claim does not refer to any nucleic acid in particular and merely reflects, albeit in a somewhat more restricted sense, the general idea of having a nucleic acid molecule which has been optimized for expression in plant tissue; an idea that a person of skill in the art would readily be able to put into practical effect by deducing an appropriate encoding sequence from D1 in view of the more specific guidance offered by D2.
Claim 3 is not obvious since neither D1 nor D2 discloses nor suggests the particular sequence referred to in the claim (SEQ ID NO:7). Given that it is disclosed that the sequence has a substantial advantage, the claim represents the selection of nucleic acids having a particular sequence from amongst the genus of all possible nucleic acids encoding the peptide and from amongst the subgenus of all possible nucleic acids employing plant optimized codons.
Date modified: October 2022
The description of an application must contain a sequence listing if the specification discloses a nucleotide or amino acid sequence, other than one that belongs to the prior art.
The sequence listing may include either, or both, English and French text matter[329] and may be different from the language of the remainder of the description. The sequence listing may also include text matter that is in a language other than English or French. It should be noted, however, that not all text matter contained in a sequence listing is necessarily considered in interpreting the scope of protection for the disclosed invention. In particular, subsection 15(3.1) of the Patent Rules states:
“If a sequence listing contains text matter that is both in English and in French, only the following version of the text matter is taken into account for the purpose of interpreting the scope of protection sought or obtained:
(a) if the sequence listing contains an indication that either English or French is the original language of that text matter, the version that is in that original language; and
(b) in any other case, the version that is in the same language as the claims.”.
When a language other than English or French is found in the language-dependent free text of a sequence listing, as per subsection 15(3.2) of the Patent Rules, that language is not taken into account for the purpose of interpreting the scope of protection sought or obtained (see also 12.02). Further, amendments to the specification and drawings of a PCT national phase application cannot be based on untranslated text matter (non-English and non-French) in the sequence listing unless that untranslated text matter is not language-dependent free text (see 20.03.02). More information on free text and language-dependent free text can be found in paragraphs 85-88 of the PCT sequence listing standard, WIPO Standard 26 (ST.26). See also 23.05.07b for an introduction to ST.26.
In some cases, the provision of a sequence listing may be needed to satisfy administrative requirements (e.g., section 58 of the Patent Rules), and to “correctly and fully describe the invention and its operation or use as contemplated by the inventor”
(i.e., subsection 27(3) of the Patent Act).
The guidance provided in 23.05.07a to 23.05.07f applies to applications filed on or after July 1, 2022. For applications having a filing date before July 1, 2022, the sequence listing may, for the purposes of section 58(1) of the Patent Rules, comply with the PCT sequence listing standard either defined in the current Patent Rules (i.e., WIPO ST.26) or as defined in the Patent Rules as they read immediately before July 1, 2022 (i.e., WIPO ST.25).[330]
Date modified: July 2022
In accordance with subsection 58(1) of the Patent Rules, “If a specification discloses a nucleotide sequence or amino acid sequence that must, in accordance with the PCT sequence listing standard, be included in a sequence listing and that is not identified as forming a part of the prior art, the description must contain the sequence listing in electronic form and both the electronic form and the content of the sequence listing must comply with the PCT sequence listing standard”
.
If prior to examination, it becomes apparent that the sequence listing on file for an application is defective, the applicant may then be given notice under section 65 of the Patent Rules to submit a sequence listing that meets the requirements set forth in section 58 of the Patent Rules, not later than three months after the date of the notice. These notices may be sent where: the sequence listing fails to comply with the PCT sequence listing standard [see 23.05.07b to 23.05.07f]; or where the applicant has failed to provide a statement indicating that the sequence listing does not go beyond the disclosure as filed as required under subsection 58(3) of the Patent Rules (see section 4.06).
If, prior to examination, it is apparent to the Office that a sequence listing was intended to be included in a regularly filed (non-PCT) Canadian application but the sequence listing was omitted, where applicable, the Office will send a notice under subsection 72(1) of the Patent Rules indicating that it may be possible to add a missing sequence listing to the application (see 3.02.05). For PCT national phase applications and divisional applications, for which a sequence listing may be missing, the Office will generally send a courtesy letter to inform the applicant.
After the examination of the application has commenced, the identification of any sequence listing defects relevant to section 58 of the Patent Rules will occur within an examiner’s report.
For example, examiners will identify defects under section 58 of the Patent Rules where:
Further, it is noted that when a sequence listing submitted in accordance with subsection 58(1) of the Patent Rules is of record in the Office, it is not permissible for a paper copy of the sequence listing to be of record. Applicants will be requisitioned to withdraw any paper copy of a sequence listing for which a PCT sequence listing standard-compliant electronic sequence listing has been made of record. Further, in accordance with subsection 58(2) of the Patent Rules, the application must not contain more than one copy of a particular sequence listing regardless of its form of presentation. For example, an application that includes a sequence listing table generally formatted according to a PCT sequence listing standard, in addition to the required electronic sequence listing, would be viewed as containing more than one copy of a particular sequence listing and, consequently, would lack compliance with subsection 58(2) of the Patent Rules.
An applicant may not request the sequence listing from another application be brought forward and recorded against the application since the Office does not consider that such a request satisfies the requirements of subsection 58(1) of the Patent Rules.
Date modified: July 2022
The term “PCT sequence listing standard”
means Standard ST.26 of the World Intellectual Property Organization (WIPO), Recommended Standard for the Presentation of Nucleotide and Amino Acid Sequence Listings using XML (eXtensible Markup Language), as amended from time to time. ST.26 is available via the WIPO website and can be found in the list of WIPO Standards.
An XML instance of a sequence listing file according to the PCT sequence listing standard (ST.26) is composed of:
The general information part contains information concerning the patent application to which the sequence listing is directed. The elements in this part include the name of the applicant and the title of the invention. The application number and filing date are also identified if known. Where priority is claimed, the earliest priority application is indicated. The general information part also contains the total number of all sequences in the sequence listing, including intentionally skipped sequences [see 23.05.07c]. Please consult paragraph 45 of the PCT sequence listing standard for more information.
The sequence data part contains one or more sequence data elements, each of which contains information about one sequence. The sequence identification number is contained within the sequence data element of a given sequence. For example, sequence identification number 1 (SEQ ID NO:1) is represented in the sequence listing as: <SequenceData sequenceIDNumber=”1”>. The sequence data elements further include various feature keys and subsequent qualifiers based on the International Nucleotide Sequence Database Collaboration (INSDC) and UniProt specifications. Each sequence must include “INSDSeq” elements for:
Please see paragraphs 50-59 of the PCT sequence listing standard for more information on the sequence data part.
The following is a simplified example of the sequence data part of a sequence listing file that describes two sequences: 1) a DNA sequence (SEQ ID NO:1), which is 14 nucleotides in length and consists of the sequence atgggtcaccttaa; and 2) a protein sequence (SEQ ID NO:2), which is 4 amino acids in length and consists of the sequence MGHL.
-<SequenceData sequenceIDNumber="1">
-<INSDSeq>
<INSDSeq_length>14</INSDSeq_length>
<INSDSeq_moltype>DNA</INSDSeq_moltype>
<INSDSeq_division>PAT</INSDSeq_division>
+<INSDSeq_feature-table>
<INSDSeq_sequence>atgggtcaccttaa</INSDSeq_sequence>
</INSDSeq>
</SequenceData>
-<SequenceData sequenceIDNumber="2">
-<INSDSeq>
<INSDSeq_length>4</INSDSeq_length>
<INSDSeq_moltype>AA</INSDSeq_moltype>
<INSDSeq_division>PAT</INSDSeq_division>
+<INSDSeq_feature-table>
<INSDSeq_sequence>MGHL</INSDSeq_sequence>
</INSDSeq>
</SequenceData>
An example of a complete sequence listing (XML file) is provided via Annex III of the PCT sequence listing standard.
Tools are available to enable patent applicants to prepare a sequence listing compliant with WIPO ST.26, as part of a national or international patent application. In particular, WIPO Sequence is a desktop application that can be downloaded or found by searching the WIPO website for “WIPO Sequence”.
Date modified: July 2022
Each nucleotide or amino acid sequence disclosed in the specification of an application, other than a sequence identified as forming a part of the prior art, must be included in a sequence listing. Each sequence in the sequence listing is assigned a sequence identification number, which is a unique number (integer). The numbers must begin with number 1 and increase in sequential order.
In cases where no nucleotide or amino acid sequence is present for a given sequence identification number, i.e., “an intentionally skipped sequence”, “000” must be used in place of a sequence – see paragraphs 58 and 59 of the PCT sequence listing standard.
Except in situations where the entire sequence listing is removed from the application, the original sequence identification number assigned to a given sequence should be maintained even after amendment of the application. Thus, in cases where a nucleotide or amino acid sequence is removed from the sequence listing by amendment, the nucleotide or amino acid sequence must be replaced with “000”. It is noted that the removal of the subject-matter from the description should not cause the application to become non-compliant with subsection 27(3) [see 23.05.07] or subsection 38.2(2) of the Patent Act [see Chapter 20 of this manual for further guidance on new matter introduced by amendments to patent applications]. Additionally it should be noted that when removing sequences from the sequence listing, any references in the specification to the associated sequence identification numbers should also be deleted.
The total number of all sequences must be indicated in the sequence listing and must equal the total number of sequence identification numbers, including all nucleotide and amino acid sequences, as well as all intentionally skipped sequences (“000”). This number is represented by the element “SequenceTotalQuantity”, which is located in the general information part of the sequence listing [see 23.05.07b].
Please consult the PCT sequence listing standard available via the WIPO website for more information about the representation of sequences.
Date modified: July 2022
If a sequence included in a sequence listing is referred to in the claims, the drawings or the part of the description other than the sequence listing, in accordance with subsection 58(4) of the Patent Rules, the reference must include the sequence identification number, as defined in the PCT sequence listing standard, preceded by "SEQ ID NO:".
Example:
In a patent application, the sequence listing included the nucleotide sequences aaatcgcgtatggg and ccctgatattaccttt, having sequence identification numbers 8 and 9, respectively. The examiner identified a defect in accordance with subsection 58(4) of the Patent Rules because the following text in the specification included reference to these sequences but failed to further identify the second sequence as SEQ ID NO:9:
“A kit comprising a first oligonucleotide primer consisting of the nucleotide sequence aaatcgcgtatggg (SEQ ID NO:8) and a second oligonucleotide primer consisting of the nucleotide sequence ccctgatattaccttt.”
.
Date modified: July 2022
Ambiguity symbols “n” and “X” can be used to define nucleotide and amino acid residues, respectively, in a sequence where a particular residue is unknown, modified, abasic or a variant. When these symbols are used in a sequence listing, they can represent only a single residue at a specific position in the sequence. Note that since such symbols represent only a single residue, a sequence of variable length must be presented by using a sufficient number of discrete symbols to represent the maximum length of the sequence.
The symbol “n” has a default value of any one of nucleotides “a”, “c”, “g” or “t/u”. The default value of symbol “X” is any one of amino acids “A”, “R”, “N”, “D”, “C”, “Q”, “E”, “G”, “H”, “I”, “L”, “K”, “M”, “F”, “P”, “O”, “S”, “U”, “T”, “W”, “Y”, or “V”. In cases where “n” or “X” represents something other than the default value in a given sequence, the corresponding nucleotide or amino acid must be further annotated in the feature table for said sequence.
The use of symbol “n” or “X” to define an “unknown” nucleotide or amino acid, respectively, is discussed in paragraphs 21 and 32 of the PCT sequence listing standard.
In the case of modified nucleotides, whenever possible, these should be represented in the sequence as the corresponding unmodified nucleotides, i.e., “a”, “c”, “g” or “t”. However, the symbol “n” must be used to represent any modified nucleotide in a sequence that cannot otherwise be represented by any other symbol in the PCT Sequence Listing Standard (see Annex I, Section 1, Table 1 for a list of nucleotide symbols to be used in a sequence) and must be further described in the feature table as provided in paragraph 17 of the PCT Sequence Listing Standard. For instance, the modified nucleotide, 4-acetylcytidine, would be represented in the sequence by the symbol “n” and would be further described in the feature table for that sequence using the abbreviation “ac4c” – see Annex I, Section 2, Table 2 of the standard for more information.
Modified amino acids, including D-amino acids, should be represented in the sequence as the corresponding unmodified amino acids whenever possible. The symbol “X” must be used to represent an amino acid that cannot otherwise be represented by any other symbol in the PCT Sequence Listing Standard (see Annex I, Section 3, Table 3 for a list of amino acid symbols to be used in a sequence) and must be further described in the feature table as provided in paragraph 30 of the PCT Sequence Listing Standard. For instance, the modified amino acid, 2-aminoadipic acid, would be represented in the sequence by the symbol “X” and would be further described in the feature table for that sequence using the abbreviation “Aad” – see Annex I, Section 4, Table 4 of the standard for more information.
Sequence variants, such as alternatives, deletions, insertions, or substitutions, are represented in the sequence listing – please see paragraphs 93-100 of the PCT Sequence Listing Standard for detailed information.
It should be further highlighted that the PCT Sequence Listing Standard now requires the following sequence features, if present, to be identified in the sequence listing:
Please consult the PCT Sequence Listing Standard for more detailed information.
The foregoing discussion relates only to the manner in which the foregoing symbols may be used as a matter of nomenclature. During examination, an examiner must consider whether or not the use of such symbols contravenes the Patent Act and/or Rules, for example on the basis of clarity [see 23.05.01].
Date modified: October 2022
If a sequence listing is found to contain errors, any correction of the listing must comply with the requirements of section 38.2 of the Patent Act. That is, no new matter may be added to the specification or drawings as originally filed and any correction made to a sequence listing must be reasonably inferable from the specification or drawings as filed except for amendments to divisional applications and amendments to applications that were originally filed containing text matter in a language other than English or French, which have further requirements, see 20.04 and 20.03, respectively. Where the corrected sequence could only be determined by, for example, re-sequencing a sample, the correction is not reasonably to be inferred.
Date modified: October 2019
Deposits of biological material are addressed in the Patent Act at subsections 38.1(1) and (2). Note that for the purposes of section 38.1, the term “biological material” may include bacteria, bacteriophages, cells in culture, hybridomas, filamentous fungi, yeasts, plant seeds, viruses, purified nucleic acid molecules, plasmids, and replication-defective cells.
Subsection 38.1(1) of the Patent Act provides that:
Where a specification refers to a deposit of biological material and the deposit is in accordance with the regulations, the deposit shall be considered part of the specification and, to the extent that subsection 27(3) cannot otherwise reasonably be complied with, the deposit shall be taken into consideration in determining whether the specification complies with that subsection.
Subsection 38.1(2) of the Patent Act provides that:
For greater certainty, a reference to a deposit of biological material in a specification does not create a presumption that the deposit is required for the purpose of complying with subsection 27(3).
Where a specification refers to a deposit, the deposit shall be considered part of the specification if it is in accordance with the regulations. Sections 93 to 98 of the Patent Rules regulate deposits of biological material. In particular, paragraph 93(1)(a) requires the deposit to be made by the applicant or their predecessor in title with an international depositary authority (IDA) on or before the filing date of the patent application. Before the application is open to public inspection at the Patent Office, the applicant must inform the Commissioner of the name of this authority and the accession number given to the deposit as per paragraph 93(1)(b). The description must include this information in order to satisfy paragraph 93(1)(c) of the Patent Rules.
Where the deposit of biological material is taken into consideration by an examiner in determining whether the specification complies with subsection 27(3) of the Patent Act [see 23.06.01 below], the examiner may requisition the applicant to amend the description to include the date the deposit was made in the IDA. The request should only be made in situations where the examiner cannot confirm the date the deposit was made in the IDA (e.g., the date of the deposit is not on record in the Patent Office and is not otherwise publically-available). In such cases, a requisition under section 94 of the Patent Rules should be identified in an examiner’s report.
Further practical aspects of the Patent Rules are covered in Appendix 1 of this chapter.
Date modified: October 2019
Bearing in mind that a specification must both adequately describe and enable an invention in order to satisfy subsection 27(3) of the Patent Act so that “when the period of monopoly has expired the public will be able, having only the specification, to make the same successful use of the invention as the inventor could at the time of his application”
,[332] sufficiency must be considered where the specification refers to a biological deposit. The considerations respecting sufficiency of disclosure as a requirement for patentability are more fully addressed in Chapter 14 of this manual.
A deposit of biological material may be made whether or not it is necessary to enable the invention as required per subsection 27(3) of the Patent Act. However, where the invention cannot be enabled in the absence of access to a biological material, the deposit is a necessary element to make the description sufficient unless the required material is publicly known and reliably available to the person skilled in the art. A biological material is considered to be reliably available if it can be obtained commercially or can be reproducibly prepared or isolated from available materials using established procedures and without undue experimentation. In the case of plant seeds, the Office considers a seed to be reliably available where it enables one to obtain, in a reproducible manner, a homogeneous population of plants that are identical to the plant of the invention.
The fact that a biological deposit has been made does not of itself mean that an invention has been adequately described.[333] A claim to a desired product does not merit protection simply because reference is made to where the product can be found. Thus, if it is possible to define the product in clear and explicit terms, a deposit is not considered a substitute for a full and correct description of the product itself and, in view of subsection 38.1(1) of the Patent Act, would not of itself meet the requirements of subsection 27(3) of the Patent Act.
Whenever possible, it is preferable that both methods of disclosure should be used[334] (i.e., disclosures relating to both the deposit of biological material and a clear and explicit description of the product or process of making the product).
Example:
The specification as filed describes both a new mutant strain of bacteria, which is useful for treating gastrointestinal disorders, and a nucleic acid molecule isolated from the strain. The description includes the dates of the original deposits with the international depositary authority and the corresponding accession numbers of both the strain and plasmid comprising the nucleic acid molecule.
Claims:
Analysis: claim 1 features a bacterium strain, which is partly defined by reference to its biological deposit number. In this case, recognizing that it is not always possible to describe the matter in terms of its structure and/or physical characteristics, a description of the biological deposit in the description provides a sufficient disclosure of the claimed strain and, therefore, satisfies subsection 27(3) of the Patent Act.
Claim 2 is directed to an uncharacterized nucleic acid molecule defined by reference to biological deposits containing the molecule. Given that it is possible to define the nucleic acid molecule in clear and explicit terms (e.g., by its DNA sequence) and despite the fact that the skilled person in the art may be able to isolate the molecule from the deposit and characterize it (e.g., determine its sequence), the mere inclusion of the deposit information in the specification is not a substitute for a full and correct description of the molecule itself. In the absence of a disclosure of the DNA sequence of the molecule in the specification, subsection 27(3) of the Patent Act is not satisfied. The claim may also be considered non-compliant with subsection 27(4) of the Act since the claimed subject-matter is not defined in distinct and explicit terms.
Date modified: October 2019
Where an invention cannot be enabled without requiring access to a biological material associated with the invention, a description may lack sufficiency unless a deposit of this material was made [see 23.06.01]. This requirement extends to an allegedly anticipatory disclosure relevant under section 28.2 of the Patent Act [see Chapter 18 for further guidance]. Consequently, if a prior art disclosure requires access to a biological material in order for the matter described therein to be practised, the biological material must necessarily have been reliably available to the person skilled in the art before the claim date in order for the disclosure to be anticipatory.
Example 1:
An application claims a mutant strain of Citrobacter sp. that is able to effectively remove mercury from wastewater. The description provides details of the biological deposit of the strain with an international depositary. A search of the prior art reveals document D1, which discloses an isolated bacterial strain of Citrobacter sp. that has an ability to degrade mercury but does not describe a biological deposit or how to otherwise obtain the strain.
Claims:
Analysis: prior art document D1 discloses a strain that falls within the scope of claim 1; however, D1 is not enabling since the strain is not reliably available to the person skilled in the art. The strain is further defined in claim 2 by reference to a particular biological deposit, which is neither disclosed nor enabled in D1. Thus, the subject-matter of the claims is not anticipated by D1. It is noted that the examiner may additionally determine that the claims are defective in view of section 60 of the Rules and/or subsection 27(4) of the Act.
Example 2:
An application discloses plasmid Y and provides details of its biological deposit with an international depositary. Prior art document D2 describes “plasmid X”, which was constructed from various known genetic elements using known methods. Plasmid X was not deposited but the genetic elements used to construct it were all freely available to the public.
Claim:
Analysis: the claim is anticipated since claimed plasmid Y is indistinguishable from known plasmid X. Further, a person of skill in the art would be enabled to construct plasmid Y using known, freely available, genetic elements and methods. The fact that the plasmids do not share the same name does not negate the finding of anticipation.
Date modified: November 2017
Antibodies, as a class of chemical compounds, have been structurally and functionally well-characterized. The structure of an antibody relates directly to its biological function, including its binding specificity and affinity to its target antigen. Structurally, each antibody is composed of light and heavy polypeptide chains where each chain has variable and constant regions. The variable regions comprise subregions involved in antigen binding, which are known as the complementarity determining regions (CDRs).
It is well established in the art that the formation of an intact antigen binding site generally requires the association of the complete heavy and light chain variable regions of a given antibody, each of which consists of three CDRs which provide the majority of the contact residues for the binding of the antibody to its target epitope. Given that the sequences of the CDRs are responsible for the specific binding of the antibody to its antigen, small changes to those sequences may significantly and unpredictably alter binding specificity and affinity. Therefore, it is generally expected that all of the heavy and light chain CDRs in their proper order and in the context of framework sequences which maintain their required conformation, are required in order to produce an antibody and that proper association of heavy and light chain variable regions is required in order to form functional antigen binding sites.
It is known that, in general, immunization of a mammal with an antigen results in the production of an antiserum containing a heterogeneous mixture of antibodies in which individual antibodies bind to different regions displayed on the surface of the immunizing antigen (i.e., an epitope or antigenic determinant). Thus, antiserum comprises an entire family of antibodies capable of binding to different epitopes on an antigen.
An antibody is often defined in functional terms by its specific binding to a particular target antigen. A claim directed to “an antibody which specifically binds to antigen X” typically represents a generic group of structurally different antibodies having common binding specificity to the antigenic target. This contrasts with a claim to a particular antibody which has been defined in terms of a property of the antibody itself rather than merely by what it binds (for example, the particular antibody is defined in terms of its encoding DNA/protein sequence, or by reference to a biological deposit that was made in accordance with the Patent Rules). Thus a claim to “an antibody which specifically binds to antigen X” is considered to be a claim to a generic group of structurally different antibodies having said binding specificity. Conversely, a claim to “an antibody which specifically binds to antigen X wherein said antibody has a heavy chain encoded by a nucleic acid of SEQ ID NO: 1 and a light chain encoded by a nucleic acid of SEQ ID NO:2” or a claim to “an antibody which specifically binds to antigen X and is produced by a hybridoma having accession number ABC-123”, encompasses only the particular antibody, i.e. is not a claim to a generic antibody.
A claim to an antibody, as with a claim to any other subject-matter, must be supported by a specification that satisfies subsection 27(3) of the Patent Act. In the case of antibodies, this means that at the relevant date, which is deemed to be the filing date[335], the specification must:
Generally, a claim to an antibody specific for antigen X will be considered supported by a specification provided:
The claims must also distinctly and explicitly define subject-matter that is novel, non-obvious, useful and statutory.
If antigen X is known or obvious in view of the prior art then an antibody reactive with that antigen would generally be considered obvious.
Where the prior art discloses and enables antibodies reactive with a close structural relative of antigen X, then a claim to an antibody reactive with antigen X (e.g. an antibody “capable of binding” or “that specifically binds” to antigen X) will be anticipated if the claim, upon a purposive construction, is construed to encompass cross-reacting antibodies of the prior art.
An antibody invention must also be useful. An inventor need not expressly set out the utility of the antibody in the specification; however, if the invention’s utility is questioned, then it must be demonstrated or soundly predicted as of the application’s filing date in order to comply with section 2 of the Patent Act [for further guidance see 23.07.05].
Example:
The description discloses a novel protein that has utility as a diagnostic target for detecting a disease caused by a pathogenic bacterium. Also disclosed are the amino acid sequence of the protein (SEQ ID NO:2), methods of purifying the protein using recombinant techniques, and reference to routine methods of preparing antibodies to a protein by immunizing a suitable mammalian host. The description is silent as regards the production of any antibodies and lacks any working examples of an antibody specific to the protein.
Claim:
1. An antibody that specifically binds to a protein consisting of the amino acid sequence set forth in SEQ ID NO:2.
Scenario 1
A search of the prior art for the sequence depicted in SEQ ID NO:2 reveals that the closest structural relative to the protein is 20% identical with no common domains of any significance.
Analysis: the claim is fully supported by a specification that satisfies subsection 27(3) of the Patent Act because the specification is enabling with respect to preparing antibodies and the scope of the claim in respect of the antigenic target is limited to the fully characterized protein of SEQ ID NO:2 which serves as a correct and full description of the corresponding antibody that specifically binds to it. Recognizing that the antigenic protein (SEQ ID NO:2) is not disclosed in the prior art, it follows that the claimed antibody, which specifically binds this protein, is novel and non-obvious. The protein (SEQ ID NO:2) itself has utility as a diagnostic target and antibodies that bind the protein serve a specific useful purpose. Further, the subject-matter of the claim is defined in distinct and explicit terms. Therefore, the claim complies with the Patent Act and Rules.
Scenario 2
A search of the prior art for the sequence depicted in SEQ ID NO:2 reveals that the protein is a low-molecular-weight member of a class of known proteins. Prior art document D1 teaches that antibodies to this class of proteins have never been prepared despite several attempts.
Analysis: the description is silent as regards the successful production of antibodies against the protein of SEQ ID NO:2. Considering that D1 discloses that, despite several attempts, antibodies have never been raised against proteins of a similar type, the person skilled in the art would not regard the instant specification as sufficient to enable the production of the claimed antibody. Thus, paragraph 27(3)(b) of the Patent Act is not satisfied. It is noted that the antibody of claim 1 is otherwise correctly and fully described by way of the disclosure of the fully characterized antigen to which it specifically binds.
Date modified: January 2017
Polyclonal antibodies can be thought of as a generic group that is representative of the entire family of antibodies in antiserum capable of binding a target antigen. Polyclonal antibodies share specificity to the target antigen yet each individual antibody can differ in regard to which epitope on the antigen it specifically binds.
Methods for preparing polyclonal sera are well known in the art and a specification generally does not need to describe in detail any of these methods to be enabling with respect to paragraph 27(3)(b) of the Patent Act.
With respect to a correct and full description of the invention pursuant to paragraph 27(3)(a) of the Patent Act, an antibody, like any other chemical compound, can be described in terms of its chemical structure; however, polyclonal antibodies are not described this way. Rather, it has become accepted practice to describe polyclonal antibodies in terms of the fully characterized antigen to which they specifically bind, e.g., “an antibody that specifically binds to antigen X”. Recognizing that an antigen is implicitly understood to carry many epitopes, a fully characterized antigen is representative of the collective of epitopes carried on the target antigen and therefore provides a correct and full description of the corresponding polyclonal binding partners.
For the purposes of paragraph 27(3)(a) of the Patent Act, a disclosure of an antigen’s chemical structure may be enough to fully characterize the antigen. Where the antigen is a protein, for instance, a description of its complete amino acid sequence is likely adequate. In some cases, a description of the antigen in other terms, such as formula, chemical name, physical properties or by biological deposit, may be adequate provided that the person skilled in the art understands the scope of the antibody claim through the unique physical or chemical properties of the antigen.
If antigen X is known or obvious in view of the prior art then polyclonal antibodies reactive with that antigen would generally be considered obvious.
A polyclonal antibody invention must also be useful (for further guidance see 23.07.05).
Date modified: January 2017
A monoclonal antibody binds to a specific epitope or antigenic determinant carried on an antigen. A monoclonal antibody can be viewed as one member of the family of polyclonal antibodies contained in antiserum produced by an immunizing antigen. For specific guidance respecting humanized and chimeric monoclonal antibodies, see 23.07.03.
Date modified: January 2017
As with claims to polyclonal antibodies, a claim to a monoclonal antibody must be supported by a specification that is both enabling and includes a correct and full description of the antibody invention. Sufficiency of disclosure is based on a fact-specific determination.[336]
The common general knowledge of the person skilled in the art is an important factor for assessing whether the specification of an application is sufficient to enable the skilled person to practise the invention. Generally, the specification need not set out a detailed procedure for producing a monoclonal antibody since the core steps for preparing a monoclonal antibody are now well known to a skilled person in the art. A description of a detailed step-by-step protocol would be necessary, however, if the invention resides, at least in part, in an applicant having inventively adapted known procedures to overcome some difficulty in making a monoclonal antibody to a particular antigen.
Although each application will be considered on its own merits, the following non-exhaustive list of factors should be considered by examiners when determining whether claims to monoclonal antibodies are enabled by a specification:
Thus, the enablement requirement of paragraph 27(3)(b) of the Patent Act is satisfied in cases where a person skilled in the art, in view of their common general knowledge and having only the specification and the fully characterized antigen, would be enabled to produce a monoclonal antibody specific to that antigen without displaying inventive ingenuity or undertaking undue experimentation.
A specification must not only be enabling with respect to a claimed monoclonal antibody but also must provide a correct and full description of the antibody to satisfy paragraph 27(3)(a) of the Patent Act.
Although each application will be considered on its own merits, the following non-exhaustive list of factors should be considered by examiners when determining whether a specification provides a correct and full description of a monoclonal antibody:
As outlined above, paragraph 27(3)(a) may be satisfied in respect of monoclonal antibodies described through reference to the fully characterized antigen to which they specifically bind.[337] Depending on the facts of the particular case, a full characterization of the antigen can entail a disclosure of its structure, formula, chemical name, or physical properties. In many cases, the disclosure of the complete amino acid sequence of an antigenic polypeptide may indicate possession of all the putative epitopes carried by the polypeptide and, by extension, serve to correctly and fully describe the genus of the corresponding generic monoclonal antibodies.[338]
Cases in which more detailed support may be required to provide a full characterization of the antibody invention include:
Depending on the facts of the particular case, this detailed support may come, for example, in the form of a disclosure of a representative embodiment of the antibody, a biological deposit, or an explicit description of the amino acid sequences of the binding regions of the monoclonal antibody, the epitope and/or the binding pocket of the target antigen essential to its function.
Date modified: January 2017
In order to be patentable, a claimed monoclonal antibody must be novel and non-obvious in accordance with sections 28.2 and 28.3 of the Patent Act, respectively. Please see Chapter 18 of this manual for a general discussion of anticipation and obviousness.
The Office considers that where the description includes a full characterization of a novel and inventive antigen X, a claim to the corresponding monoclonal antibody having specific binding to X would be novel and non-obvious.
An enabling prior art disclosure of a monoclonal antibody specific to antigen X would anticipate a claim to a generic monoclonal antibody specific to antigen X. In cases where antigen X is disclosed and enabled by the prior art, a claim to a generic monoclonal antibody that binds antigen X would be obvious in view of the prior art. However, a claim to an antibody that binds antigen X may be novel and non-obvious where the claimed antibody is additionally defined in the claim by properties that distinguish the antibody from both generic and prior art antibodies, which may include:
A monoclonal antibody invention must also be useful (for further guidance see 23.07.05).
Where an application claims nucleic acids or polypeptides relating to antibodies of the invention (e.g., light and heavy chains, variable regions, CDRs, etc.), the nucleic acids and polypeptides must be fully supported by the description (for further guidance see 23.05).
Date modified: January 2017
The following hypothetical examples are provided to help clarify the foregoing.
Example 1:
An application discloses a novel tyrosine kinase protein, its complete amino acid sequence (SEQ ID NO:2) and corresponding nucleic acid sequence (SEQ ID NO:1). According to the description, enhanced activity of the protein is associated with pulmonary fibrosis. An embodiment of the invention includes monoclonal antibodies that specifically bind and inhibit the protein although no working examples of an antibody are described. A search of the prior art failed to identify any proteins with significant identity over the full length of the amino acid sequence depicted in SEQ ID NO:2 or any corresponding nucleic acid molecules.
Claims:
Analysis: in this case, the examiner determined that claim 1 is compliant with the Patent Act and Rules (see 23.05 for further guidance on subject-matter related to this claim). The claimed subject-matter is novel and non-obvious because the prior art does not disclose or suggest any protein having an amino acid sequence with significant identity to SEQ ID NO:2. Further, the matter is fully supported by a specification that satisfies subsection 27(3) of the Patent Act because the specification is enabling with respect to preparing the protein and includes a full characterization of this protein (i.e., through the disclosure of its complete amino acid sequence). The claim also complies with subsection 27(4) of the Act as the subject-matter is distinctly and explicitly defined.
Regarding claim 2, novelty and inventiveness is acknowledged because the antigenic target of the claimed monoclonal antibody (i.e., the protein of SEQ ID NO:2) is novel and non-obvious. The scope of claim 2 in respect of the antigenic target is limited to the fully characterized protein of SEQ ID NO:2 and the examiner considers that this provides a correct and full description of the corresponding claimed monoclonal antibodies. In this case, the person skilled in the art is also enabled to produce the monoclonal antibody at the filing date of the application. Therefore, the claimed monoclonal antibody is fully supported by a specification that satisfies subsection 27(3) of the Patent Act. The claim also complies with subsection 27(4) of the Act as the subject-matter is distinctly and explicitly defined.
Example 2:
The description discloses the production of murine monoclonal antibody, M1, specific for the RF protein for use in diagnosing Rheumatoid arthritis. Also disclosed are details of a biological deposit of the hybridoma that produces the antibody. A further embodiment includes monoclonal antibodies that compete with M1 although a working example of competing antibodies is not disclosed. A search of the prior art identified the murine RF protein and its full amino acid sequence.
Claims:
Analysis: claim 1 is obvious. The scope of the claim encompasses any monoclonal antibody that is specific to the antigenic RF protein. Given that techniques for preparing monoclonal antibodies were well established as of the claim date of the application, in this case, no inventive ingenuity is required on the part of the person skilled in the art to prepare a monoclonal antibody, or antigen-binding fragment thereof, with specific binding to the known RF protein. Therefore, the claim is not in accordance with section 28.3 of the Patent Act. It is noted that the subject-matter of the claim is otherwise novel, defined in distinct and explicit terms and supported by a specification that satisfies subsection 27(3) of the Act. The scope of claim 1 in respect of the target antigen is limited to the known and fully characterized antigenic RF protein and the examiner considers that this provides a correct and full description of the corresponding monoclonal antibodies and fragments thereof. In this case, the person skilled in the art, in view of their common general knowledge of routine antibody methods and having only the specification and the fully characterized antigen, would be enabled to produce an antibody (and fragments thereof) specific to RF without displaying inventive ingenuity or undertaking undue experimentation.
Claim 2 defines the antibody by reference to a deposit of the hybridoma that produces it. The claim is novel since the prior art does not describe or enable the antibody (or antigen-binding fragment thereof) obtained from the hybridoma and is non-obvious because, unlike claim 1 to a generic antibody, claim 2 is limited to the particular antibody produced by the hybridoma having accession number IDAC 022612-11. Further, claim 2 is supported by a specification that satisfies subsection 27(3) of the Patent Act because it is enabling with respect to the particular antibody claimed and, assuming that the hybridoma which produces M1 was deposited in accordance with the Patent Rules, the provision of the deposited hybridoma serves to provide a correct and full description of the M1 antibody. Therefore, the claim fully complies with the Patent Act and Rules.
In claim 3, the antibody is distinctly and explicitly defined as one that competes with monoclonal antibody M1 for specific binding to the RF protein and, thus, satisfies subsection 27(4) of the Act. As noted above, the M1 antibody produced by the hybridoma having accession number IDAC 022612-11 is novel and non-obvious and it follows that an antibody that competes for specific binding with that particular antibody is also novel and non-obvious. Appreciating that the person skilled in the art could identify competing antibodies without undertaking undue experimentation or the need to exercise inventive ingenuity (e.g., by using routine competition binding assays), the subject-matter of claim 3 is enabled. Assuming that the hybridoma which produces M1 was deposited in accordance with the Patent Rules, the provision of the deposited hybridoma serves to provide a correct and full description of the M1 antibody and antibodies in general that specifically bind the same epitope, i.e., competing antibodies. Therefore, the claim is supported by a specification that satisfies subsection 27(3) of the Patent Act and complies fully with the Patent Act and Rules.
Claim 4 is not compliant with the Patent Act and Rules. The description discloses details of a biological deposit of the hybridoma that produces the antibody but does not disclose the full nucleotide or amino acid sequences of the antibody itself. Therefore, the polynucleotide of claim 4 lacks compliance with paragraph 27(3)(a) of the Act. It is noted that a deposit of biological material is not a substitute for a full and correct description of the polynucleotide molecule itself (see 23.06.01 for further guidance). Further, the claim lacks compliance with subsection 27(4) of the Act because the polynucleotide is not distinctly and explicitly defined in the claim.
Date modified: January 2017
Advances in genetic engineering techniques have permitted the production of therapeutic humanized and chimeric monoclonal antibodies that combine non-human (e.g., mouse) and human amino acid sequences. The antibodies retain the non-human antigen binding characteristics conferred by the non-human sequences but beneficially elicit less antibody immunogenicity in human recipients as compared to a fully non-human monoclonal antibody.
A humanized monoclonal antibody is a “CDR-grafted” antibody meaning that only the non-human complementarity determining regions (CDRs) of the variable light and heavy chains and selected variable region framework residues have been transferred or “grafted” onto a human antibody template.
A chimeric monoclonal antibody is considered by the person skilled in the art to be a monoclonal antibody in which the non-human constant regions have been replaced with human constant regions. Chimeric antibodies are generally understood to exclude CDR-grafted antibodies.
A determination of whether a specification complies with subsection 27(3) of the Act in relation to humanized and chimeric monoclonal antibodies will generally rely on the same considerations as for monoclonal antibodies [see 23.07.02a].
Recall that compliance with paragraph 27(3)(b) of the Act requires the person skilled in the art to be enabled to make or use the antibody invention. Although core steps for preparing humanized and chimeric antibodies are now well established in the state of the art, the examiner must carefully consider, on a case-by-case basis, whether the skilled person, in view of their common general knowledge in the relevant art and the teachings of the specification, was enabled to prepare a humanized or chimeric antibody specific for the target antigen without having to undertake undue experimentation or display inventive ingenuity at the filing date.
Thus, paragraph 27(3)(b) may be satisfied in cases where, at the filing date, a person skilled in the art, in view of their common general knowledge and having only the specification and a fully characterized target antigen would not have to undertake undue experimentation or display inventive ingenuity to produce a generic humanized or chimeric monoclonal antibody specific to the target antigen.
Recall also that in order to satisfy paragraph 27(3)(a) of the Act, the specification must correctly and fully describe the antibody invention. When determining whether a humanized or chimeric antibody is correctly and fully described, the examiner may rely on the same considerations as for monoclonal antibodies as outlined in 23.07.02a. In brief, depending on the facts surrounding a particular case, a humanized or chimeric antibody may be correctly and fully described through reference to, for example:
In some cases a correct and full description of a claimed humanized or chimeric antibody may require more detailed support (see 23.07.02a).
Even where subsection 27(3) of the Act is satisfied, a claim to a humanized or chimeric antibody may not be patentable if the antibody lacks novelty or inventiveness. For instance, a claim to a generic humanized monoclonal antibody may be anticipated and/or obvious in view of an enabling prior art disclosure of: the fully characterized antigenic target to which the claimed antibody binds; monoclonal antibodies (including humanized or chimeric monoclonal antibodies) specific to the same antigenic target; or nucleotide or amino acid sequences corresponding to the CDRs of the claimed antibody.
A humanized or chimeric monoclonal antibody invention must also be useful (for further guidance see 23.07.05).
Example:
An application discloses a Sonic Hedgehog (Shh) protein homolog and its complete amino acid sequence (SEQ ID NO:2). According to a working example in the description, a murine monoclonal antibody was prepared using conventional methods and was shown to have high affinity to the homolog in vitro with no cross-reactivity to other Shh proteins. The specification does not include any details of either the structure of the antibody or any hybridoma clone. The description further states that the invention encompasses antibodies specific to the Shh homolog including polyclonal, monoclonal, chimeric and humanized antibodies as well as antigen binding fragments (Fab, Fab’, F(ab’)2, scFV and diabodies), which can be obtained using routine techniques known to persons skilled in the art.
Claim:
1. An isolated antibody or antibody fragment thereof that specifically binds to a Sonic Hedgehog protein homolog comprising the amino acid sequence of SEQ ID NO:2, wherein the antibody or antibody fragment thereof is selected from the group consisting of polyclonal, Fab, Fab’, F(ab’)2, monoclonal, chimeric, scFV, diabody and humanized.
Analysis: claim 1 encompasses polyclonal antibodies, monoclonal antibodies, chimeric monoclonal antibodies, humanized monoclonal antibodies and antibody fragments (Fab, Fab’, F(ab’)2, scFv or diabody).
With respect to enablement pursuant to paragraph 27(3)(b) of the Patent Act, all of the antibodies and fragments encompassed by claim 1 are enabled since core methods for preparing these were well known to the person skilled in the art at the filing date of the application. The description also confirms that conventional methods were sufficient to at least make a monoclonal antibody specific to the homolog. A correct and full description of the subject-matter pursuant to paragraph 27(3)(a) of the Patent Act over the entire scope of claim 1 is provided by virtue of the fully characterized target antigen to which the antibodies and fragments specifically bind. In this case, the complete amino acid sequence (SEQ ID NO:2) serves to fully characterize the antigen, and by extension, the corresponding antibodies and antigen binding fragments thereof.
The examiner also determines that the target antigen is novel, non-obvious and useful and, therefore, the claimed antibodies and antigen binding fragments that specifically bind this antigen are likewise novel, non-obvious and useful. The claim also complies with subsection 27(4) of the Act as the subject-matter is distinctly and explicitly defined.
In view of the above, claim 1 complies with the Patent Act and Rules.
Date modified: January 2017
Unlike chimeric and humanized monoclonal antibodies (see 23.07.03), human antibodies are derived entirely from human genes and, in view of this, are more desirable for use in therapeutic and diagnostic applications in humans.
A determination of whether a specification complies with subsection 27(3) of the Act in relation to human monoclonal antibodies will generally rely on the same considerations as for monoclonal antibodies (see 23.07.02a).
Recall that compliance with paragraph 27(3)(b) of the Act requires the person skilled in the art to be enabled to make or use the antibody invention. Although core methodologies, such as phage display technologies and transgenic-mouse technologies, are now routinely practised by persons skilled in the art to prepare fully human monoclonal antibodies to desired antigenic targets, the examiner must carefully consider, on a case-by-case basis, whether the skilled person, in view of their common general knowledge in the relevant art and the teachings of the specification, was enabled to prepare an antibody without having to undertake undue experimentation or display inventive ingenuity at the filing date. See also 23.07.02a.
Thus, the enablement requirement of paragraph 27(3)(b) of the Patent Act is satisfied in cases where a person skilled in the art, in view of their common general knowledge and having only the specification and the fully characterized antigen would be enabled to produce the antibody specific to that antigen without displaying inventive ingenuity or undertaking undue experimentation.
Recall also that in order to satisfy paragraph 27(3)(a) of the Act, the specification must correctly and fully describe the antibody invention. When determining whether a human monoclonal antibody is correctly and fully described, the examiner may rely on the same considerations as for monoclonal antibodies as outlined in 23.07.02a.
Even where subsection 27(3) of the Act is satisfied, a claim to a human monoclonal antibody may not be patentable if the antibody lacks novelty or inventiveness. For instance, a claim to a generic human monoclonal antibody may be anticipated by an enabling prior art disclosure, or may be obvious in view of an enabling prior art disclosure of: the fully characterized antigenic target to which the claimed antibody binds; monoclonal antibodies specific to the same antigenic target; or nucleotide or amino acid sequences corresponding to the CDRs of the claimed antibody.
A human monoclonal antibody invention must also be useful (for further guidance see 23.07.05).
Date modified: November 2017
An antibody invention must also be useful in order to satisfy section 2 of the Patent Act. The utility does not need to be expressly set out in the specification; however, if the invention’s utility is questioned, utility must be demonstrated or soundly predicted as of the application’s filing date. The threshold that must be proven to establish utility is generally quite low;[341] a “mere scintilla” of utility will suffice.[342]
The skilled person in the art would generally accept that if an antigen itself has a practical utility then antibodies that bind the antigen would have at least some utility (e.g., for in vitro applications such as immunohistochemistry, flow cytometry and Western blotting). Where the subject-matter of the invention is directed to an antibody that is useful for an in vivo therapeutic application, the therapeutic utility would need to be either demonstrated or soundly predicted in order to satisfy section 2 of the Patent Act.
In cases where the utility requires the antibody to possess not only binding capacity to the target antigen but also functional activities, such as antagonist (i.e., blocking), agonist (i.e., activating) or neutralizing activity, the description would likely require more than a disclosure of the binding capacity to the target antigen to establish utility. The provision of a working example of the claimed antibody and in vitro or in vivo data showing the antibody has the required activity may be sufficient to demonstrate utility. In the absence of demonstration, the applicant must be in a position to soundly predict the additional functional activity necessary for the utility.
Date modified: March 2016
The Office considers a synergistic combination to be one in which the combined use of two or more compounds or products generates a result that is greater than the sum of its parts and provides an unexpected advantage.[343] Please see sections 14.04.03 and 16.07 of this manual for a general discussion of combinations.
Generally, implementing the physical acts of mixing or physically combining different chemical compounds or products does not require inventive activity; however, an inventive step may be acknowledged for a synergistic combination of known components that leads to an unexpected advantage (i.e., the synergistic effect) provided the advantage was disclosed in the originally filed description.[344]
To ascertain whether an unexpected advantage has been produced by a combination, it is necessary to be aware of the point of reference (the result to be expected from combining the individual components), either in view of the common general knowledge of the person skilled in the art in the relevant field or in view of the description.
The utility of a chemical combination is typically closely associated with the unexpected advantage. The utility of the combination must be established at or before the filing date of the application over the entire scope of the claim. Thus, where a synergistic effect is explicitly promised in an application, the synergistic effect must be either demonstrated or soundly predicted in order to establish utility.
In cases where a first compound has been applied to its known purpose and another compound in the combination unexpectedly enhances the result of the first compound, the enhancement effect is, in some fields, referred to as potentiation and requires similar considerations to those described above with regard to patentability.
Date modified: October 2019
A “reach-through” claim seeks to encompass subject-matter extending beyond the described invention in cases where the matter has not yet been identified by the inventor but may be discovered through future use of the invention. Considering that “nothing that has not been described may be validly claimed”
,[345] in a reach-through claim the subject-matter defined by the claim is not supported by the specification since the specification fails to provide an adequate written description of the matter.
To illustrate, consider an invention featuring a novel and inventive protein associated with disease Y. Claims to the protein and a method of screening for drugs that inhibit the protein may be acceptable; however, a claim to a product defined by the screening method, e.g. “a drug identified by the method of claim 2” would be considered a reach-through claim where products of the method have not yet been identified. In effect, the claim to a product identified by the method attempts to “reach through” the method in order to define a product that could be potentially identified in the future. Therefore, unidentified products of the method cannot be claimed as such a claim would fail to satisfy section 60 of the Patent Rules. Furthermore, where a product is claimed and not properly described in the specification, the disclosure and enablement requirements of subsection 27(3) of the Patent Act cannot be satisfied.
As a further example, consider an invention directed to a new and inventive method of identifying receptor ligand antagonists. Although such a method may be patent-eligible, the method cannot be legitimately extended to generally claim all antagonists which might eventually be discovered through the future use of the inventive method. Likewise, the subsequent use of these unidentified antagonists, e.g. to treat disease, would not be patentable.
Thus, examples of reach-through claims may include:
Date modified: October 2019
For the purposes of subsection 38.1(1) of the Patent Act, the term “biological material” includes material which is capable of direct or indirect self-replication. Directly self-replicating biological materials are those that replicate by themselves. Indirectly self-replicating biological materials are those that are capable of replication only in association with a directly self-replicating biological material. Bacteria, fungi (including yeast), plant seeds, cells in culture and hybridomas are representative examples of directly self-replicating materials; indirectly self-replicating materials include nucleotide sequences, plasmids, vectors, viruses, phages and replication-defective cells.
Date modified: October 2019
The Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure (The Budapest Treaty) was established in 1977. The Treaty is administered by WIPO and obliges contracting states to recognize the fact and date of a deposit of biological material for patent purposes, when it is made in a depositary which has acquired official status under the Treaty. Such a depositary is known as an International Depositary Authority (IDA). An applicant who is making multiple patent filings need only make one IDA deposit to satisfy the deposit practice in all contracting states.
The term “microorganism” is not defined in the Treaty so that it may be interpreted in a broad sense as to the applicability of the Treaty to microorganisms to be deposited under it. Whether an entity technically is or is not a microorganism matters less in practice than whether deposit of that entity is necessary for the purposes of disclosure and whether an IDA will accept it. Thus, for example, tissue cultures, plant seeds and plasmids can be deposited under the terms of the Treaty, even though they are not microorganisms in the strict sense of the word.
The Budapest Treaty came into force, with respect to Canada, on September 21, 1996.
Date modified: October 2019
A list of International Depositary Authorities and their specific requirements is available at the WIPO website.
Date modified: October 2019
In accordance with paragraph 93(1)(a) of the Patent Rules, a deposit of biological material with an international depositary authority must be made on or before the filing date of the application.
Date modified: October 2022
In accordance with paragraph 93(1)(b) of the Patent Rules, the applicant must, before the day on which the application becomes open to public inspection at the Patent Office, inform the Commissioner of the name of the IDA and the accession number given by the IDA to the deposit. In accordance with subsection 93(1.1), for a PCT national phase application that is published by WIPO on or before the national phase entry date, the requirement in paragraph 93(1)(b) is considered to be met only if the information referred to in that paragraph was furnished in accordance with the requirements of the Patent Cooperation Treaty before the day on which the international application is published. This information must be included in the description as required by subsection 93(1)(c) of the Patent Rules.
In cases where a deposit referred to in the specification is taken into consideration by an examiner in determining whether the specification complies with subsection 27(3) of the Patent Act, the examiner, in accordance with section 94 of the Patent Rules, may requisition the applicant to amend the description to include the date of the deposit in the IDA if the date was not already included in the description [see 23.06 for specific conditions].
Date modified: October 2019
When a sample of biological material is deposited in an IDA under the Budapest Treaty for the purposes of patent protection, the depositor undertakes not to withdraw the sample for a period of at least 30 years from the date of deposit and for at least five years from the date of the most recent request made to the depositary for the furnishing of a sample of the deposited material (Rules 6 and 9 of the Regulations under the Budapest Treaty).
Date modified: October 2022
After an original sample of biological material has been deposited in an IDA (an original IDA deposit), circumstances may necessitate that a new sample of the same material be deposited in either the same or a different IDA (Article 4 of the Budapest Treaty) or that the sample be transferred to a substitute IDA (Rule 5 of the Regulations Under the Budapest Treaty).
If an IDA cannot furnish a sample of deposited material because it is no longer viable, a depositor must make a new deposit in the same IDA.
If an IDA cannot furnish a sample of deposited material because the sample must be sent abroad and this is prevented by export or import restrictions, a depositor may make a new deposit in another IDA.
To maintain an original IDA deposit date, paragraph 93(1)(e) requires that a new deposit be made in accordance with Article 4 of the Budapest Treaty in the case where the depositor is notified under Article 4 of the inability of the IDA to furnish samples for any reason, particularly where the sample is no longer viable or cannot be sent abroad, or that the IDA's status has changed. The new deposit must be made within three months after the date on which the depositor received the notification (Article 4(1)(d)) and must be accompanied by a statement that the newly deposited material is the same as that originally deposited (Article 4(1)(c)).
If an IDA temporarily or permanently discontinues any of the tasks required of it as an IDA such that samples of deposited biological material can no longer be provided, the defaulting IDA is required to transfer samples of deposited materials to another IDA. The new IDA is referred to as a substitute IDA and the deposit is known as a substitute deposit.
In accordance with the Patent Rules, whenever a deposit of a biological material is made with another IDA under Article 4(1)(b)(i) or (ii) (paragraph 93(1)(f) of the Patent Rules) or transferred to a substitute IDA under Rule 5 of the Budapest Treaty (paragraph 93(1)(d) of the Patent Rules) the applicant or patentee must inform the Commissioner of the accession number given by the new or substitute IDA to the deposit not later than three months after the date of issuance of a receipt by that IDA. If the application is a PCT national phase application, the applicant must inform the Commissioner before the later of three months after the date of issuance of a receipt by that IDA and three months after the national phase entry date of the application.
Date modified: January 2025
Deposited biological material becomes available to the public once a patent application is open to inspection under section 10 of the Patent Act, or for applications filed before October 1, 1989 once a patent issues.
In accordance with subsection 95(1) of the Patent Rules, an applicant is entitled to restrict access to a deposit of biological material until such time as a patent has issued, or the application is refused, withdrawn, or deemed to be abandoned and beyond the period of reinstatement provided the applicant submits a request to the Commissioner before the application is open to public inspection at the Patent Office. In such cases, any person may request that an independent expert be nominated by the Commissioner in accordance with subsection 96(1) of the Patent Rules. Once so nominated, that expert will be able to obtain access to the deposit in accordance with the Patent Rules.
In order to access a deposited biological material, a request must be made to the international depositary authority (IDA). Where a restriction on access has been made by the applicant and is in effect, only a person authorized by the depositor or the independent expert may make such a request (subsection 98(1) of the Patent Rules). When such a restriction is not in place, or no longer applicable, any person may request access to the deposited material.
When the biological material is referred to in a pending patent application, IDAs may require the patent office where the pending application has been filed to provide a certification before the biological material can be accessed. In such cases, in Canada, a request for a sample of the biological material must be submitted to the Commissioner of Patents and requires, inter alia, that the requester undertake in accordance with paragraph 97(2)(b) of the Patent Rules not to make the sample, or any material derived from the sample, available to any other person nor to use the sample, or any material derived from the sample, for any purpose other than experiments that relate to the subject-matter of the application until such time as a patent issues, or the application is refused, withdrawn or deemed to be abandoned and beyond the period of reinstatement.
In the case of a patent that has been issued, or an application that has been refused, withdrawn or deemed abandoned and beyond the period of reinstatement, the request for a sample of the deposited material may be made directly to the IDA without the need to provide a request form certified by the Commissioner of Patents unless the IDA specifically requires that a certified request form be submitted. Should a certified request form be required, the requester must fill and send the request form to the Patent Office for certification.
Request form WIPO BP/12 for the furnishing of a sample of deposited material is published on the website of the Canadian Intellectual Property Office and is also provided in Appendix 3 of the Guide to the Deposit of Microorganisms under the Budapest Treaty which may be found on the WIPO website.
Detailed procedures for obtaining samples of biological materials are provided in Appendix 2 (see 23.11).
Date modified: October 2019
In accordance with subsection 96(1) of the Patent Rules, the Commissioner will nominate an independent expert on the request of any person and with the agreement of the applicant. Both the applicant and the person requesting that an expert be nominated may make suggestions as to who would be a suitable expert. In the event that the Commissioner and the applicant cannot agree on the nomination of an independent expert, the request under subsection 95(1) of the Patent Rules, i.e., that the Commissioner only authorize the furnishing of a sample of the deposited material to the nominated independent expert, is deemed never to have been submitted in view of subsection 96(2) of the Patent Rules.
Date modified: October 2019
After a request form for the furnishing of a sample of deposited biological material has been filed with the Commissioner of Patents [see 23.10.07], the Commissioner will, in accordance with subsection 97(2) of the Patent Rules, make the certification referred to in Rule 11.3(a) of the Regulations Under the Budapest Treaty that the deposit is referred to in an application for patent in Canada, that the requester has fulfilled all conditions for the furnishing of a sample, and that the requester has a right to a sample of the deposited material.
A copy of the request along with the certification is then sent to the requester in accordance with subsection 97(3) of the Patent Rules or in the case where the requester is an independent expert, to the applicant and to the person who requested the nomination of the expert in accordance with subsection 98(2) of the Patent Rules.
Date modified: January 2025
To obtain a sample of a biological material referred to in a pending Canadian patent application on which no restriction has been placed under subsection 95(1) of the Patent Rules:
To release a sample of a biological material referred to in a pending Canadian application, on which a restriction has been placed under subsection 95(1) of the Patent Rules, to an independent expert:
To obtain a sample of a biological material referred to in an issued patent, or a patent application that has been refused, withdrawn or deemed to be abandoned and beyond the period of reinstatement:
Date modified: October 2019
As per section 34.1 of the Patent Act, any person may file prior art with the Commissioner. This prior art can consist of patents, applications for patents open to public inspection and printed publications that the person believes have a bearing on the patentability of any claim in a patent application. Prior art filed under section 34.1 of the Patent Act must be accompanied by an explanation of why the art is pertinent. Please note that submissions of prior art must follow the general written communications requirements of the Patent Rules. Please see section 2.02 for more information.
In accordance with section 12 of the Patent Rules, any written communication submitted to the Commissioner under section 34.1 of the Patent Act, will be acknowledged by the Commissioner. The provider will be notified that the filing of prior art has been received but will not be directly informed regarding any resulting action taken. The examiner will not discuss the prosecution of the application with the provider; however the provider has access to the prosecution file of the application at the time the file is opened to public inspection. The prior art is made part of the application file and the applicant is notified that a submission of prior art has been made.
If the application referred to by the person submitting the prior art is a PCT application which has not yet entered the national phase in Canada, the Canadian Patent Office will retain the submission until the date for late national entry in Canada has passed.
Date modified: October 2019
In accordance with section 12 of the Patent Rules, any written communication submitted to the Commissioner before the granting of a patent with the stated or apparent intention of protesting against the granting of that patent, will be acknowledged by the Commissioner. Please note that protests must follow the general written communications requirements of the Patent Rules. Please see section 2.02 for more information. The protestor will not be directly informed regarding any resulting action taken; however a protestor has access to the prosecution file of the application at the time the file is opened to public inspection.
The protestor should identify the Canadian patent application number if possible or at the very least, the inventor or applicant to allow the Office to identify the appropriate patent application. Any protest that fails to identify an application by number, inventor or applicant reduces the likelihood of the Patent Office locating the application and therefore reduces the effectiveness of the protest.
When a protest does not identify an application by number, the Patent Office carries out a search to identify the application to which the protest applies. If the application is found, the protest is made part of the application file and the applicant is notified of the protest. As detailed above, the protestor will also be advised of the receipt of the protest in the Patent Office; however, the application number will not be disclosed if this application is not already laid open for public inspection. When a specific application cannot be located (e.g. when the application has not yet been filed at the Patent Office or when there is not enough information in the protest to identify the application), the Patent Office will retain the protest for two years during which time the Office will continue to attempt to identify the relevant application.
Date modified: May 2014
A protest or a filing of prior art is only considered by the patent examiner after examination of the application has been requested. Information in a protest or a filing of prior art is taken into account by the examiner, and will be used during prosecution if it is found to be pertinent. In the event that a notice of allowance has been sent to the applicant but the patent has not yet issued, the pertinence of the protest or the filing of prior art will determine whether the notice of allowance will be withdrawn. Where the protest or filing of prior art calls the patentability of the application into question, the Notice of Allowance will be withdrawn and the application will be returned to the examiner for further consideration. See chapter 25 for more information on notice of allowance and withdrawal thereof.
A protest may contain affidavits. An affidavit may contain information that could raise serious questions as to whether or not a patent should be granted or lead to documentation that could be pertinent. A protest containing an affidavit should support any allegations with dated material or give details to help locate such material. Affidavits containing allegations which are not supported by dated documentation will usually be disregarded.
Date modified: June 2016
Any protest or filing of prior art will become part of the laid-open application file and will therefore be made available to the public. Should a party filing a protest or a filing of prior art request that the protest or filing of prior art remain confidential, the protest or filing of prior art will be returned to the sender and will not be considered by the patent examiner. Parties filing a protest or filing of prior art should note that they cannot remain anonymous; information identifying the protestor, such as that provided in the protest or filing of prior art cover letter, will be made available to the public.
Date modified: October 2022
When an examiner has reasonable grounds to believe that a patent application complies with the requirements of the Patent Act and Patent Rules, the Commissioner will notify the applicant under subsection 86(1) of the Patent Rules, via a notice of allowance, that the application has been found to be allowable.
In the notice, the Commissioner will require the applicant to pay the final fee (see CIPO’s website on Patent Fees) no later than four months after the date of the notice. If the final fee is not paid within the four-month period, the application will be deemed abandoned under paragraph 132(1)(f) of the Patent Rules.
The final fee is composed of the basic fee, a fee for each page of the specification and drawings in excess of 100 other than pages of a sequence listing submitted in electronic form, and an excess claim fee[346] for each claim in excess of 20 that was not already paid when requesting examination.
To calculate the excess claim fee, the Office will look at the maximum number of excess claims in the application from the date of the request for examination until the application is allowed.
For example, in the following scenario, the applicant would have to pay the fee for 4 excess claims to comply with the notice of allowance:
The maximum number of claims in the above example is 30. This means that the applicant must pay for 10 additional claims. Since the applicant already paid for 6 excess claims, the notice of allowance will require the applicant to pay for 4 additional excess claims. Where the examiner approves an application for allowance following the review of the response following a rejection under subsection 86(5) of the Patent Rules (i.e. Final Action), the Commissioner will notify the applicant, via notice of allowance, that the rejection is withdrawn and the application has been found to be allowable under subsection 86(6) of the Patent Rules (a notice of allowance). The applicant is required to pay the final fee (see CIPO’s website on Patent Fees) not later than four months after the date of the notice. If the final fee is not paid within the four-month period, the application will be deemed abandoned under paragraph 132(1)(f) of the Patent Rules.
Date modified: June 2023
Where an examiner has reasonable grounds to believe that the application contains only certain minor defects, and if not for those defects, the application would otherwise comply with the Patent Act and Patent Rules, the Commissioner will notify the applicant under subsection 86(1.1) of the Patent Rules, via a conditional notice of allowance. The conditional notice of allowance will provide an indication to the applicant that the application will be found allowable if the outstanding minor defects are satisfactorily addressed with amendments or arguments. Examples of minor defects that could be included in a conditional notice of allowance:
In the notice, the Commissioner will require the applicant to make the amendments to address the defects set out in the conditional notice of allowance and pay the final fee (see CIPO’s website on Patent Fees) no later than four months after the date of the notice.
The final fee is composed of the basic fee, a fee for each page of the specification and drawings in excess of 100 other than pages of a sequence listing submitted in electronic form, and an excess claim fee[347] for each claim in excess of 20 that was not already paid when requesting examination.
To calculate the excess claim fee, the Office will look at the maximum number of excess claims in the application from the date of the request for examination until the application is allowed.
For example, in the following scenario, the application would have to pay the fee for 4 excess claims to comply with the fee component of the conditional notice of allowance:
The maximum number of claims in the above example is 30. This means that the applicant must pay for 10 additional claims. Since the applicant already paid for 6 excess claims, the conditional notice of allowance will require the applicant to pay for 4 additional excess claims.
If the applicant does not reply in good faith to the conditional notice of allowance and the final fee is not paid within the four-month period, the application will be deemed abandoned under paragraph 132(1)(g) of the Patent Rules.
Date modified: October 2022
Examination ceases once a notice of allowance has been sent. Amendments after a notice of allowance is sent are not permitted as per subsection 100(1)(a) of the Patent Rules, unless: the notice of allowance is withdrawn (see section 25.02), or the amendment corrects an obvious error under subsection 100(2) of the Patent Rules (see section 25.01.03).
Amendments after a conditional notice of allowance is sent are not permitted as per subsection 100(1)(a), unless: the amendments are limited to those that address the defects identified in the conditional notice of allowance (paragraph 100(1)(b) of the Patent Rules); or those that correct obvious errors (subsection 100(2) of the Patent Rules) (see section 25.01.03).
Where an impermissible amendment is submitted after a conditional notice of allowance is sent, the notice will be withdrawn (subsection 86(15) of the Patent Rules), and the application will be examined without taking into account the impermissible amendments and any other amendments made from the day the conditional notice of allowance was sent to the day the conditional notice of allowance was withdrawn (subsection 100(3) of the Patent Rules).
Date modified: October 2022
An applicant may amend obvious errors in the specification and drawings after a notice of allowance has been sent if the amendment is submitted on or before the date of the payment of the final fee under subsection 100(2) of the Patent Rules.
An applicant may amend obvious errors in the specification and drawings after a conditional notice of allowance has been sent if the amendment is submitted on or before the date on which the applicant replies in good faith to the conditional notice of allowance.
The basis for obviousness of the error are the specifications and drawings contained in the application on the day the notice of allowance or the conditional notice of allowance was sent. The amendment is permissible if it is obvious that something other than what appears in the specification and the drawings was intended and that nothing other than the proposed amendment could have been intended, although the final determination of obviousness will be determined by the examiner. There is no fee for the correction under this subsection.
The mechanism for the correction of obvious errors does not include the replacement for “better quality” pages of the specification or drawings.
Pages otherwise identical to the current pages, but replacing inferior quality ones and that have no pre-existing defect that would have been identified in a previous examiner’s report would be considered as an amendment, rather than as a correction of an obvious error, and, as such, cannot be accepted, pursuant to subsection 100(1) of the Patent Rules.
Date modified: October 2022
During the course of normal operations and prosecution, a notice of allowance may be withdrawn under subsection 86(14) of the Patent Rules.
A conditional notice of allowance can be withdrawn under subsection 86(14.1) or subsection 86(15) of the Patent Rules. Applicants can also request that a notice of allowance or a conditional notice of allowance be set aside by requesting continued examination (see section 11.04.02c).
Date modified: October 2022
The Commissioner will withdraw a notice of allowance under subsection 86(14) of the Patent Rules before a patent is issued if the examiner has reasonable grounds to believe that the application does not comply with the Patent Act and the Patent Rules, except when the defects would not affect the readability, clarity or validity of the patent, if it were granted. The notice of allowance will not be withdrawn if the application is deemed abandoned.
An application may be withdrawn from allowance, for example, in view of applicable prior art identified in a protest or in a filing of prior art under section 34.1 of the Patent Act.
The Commissioner will inform the applicant by notice that the notice of allowance has been withdrawn and refund the final fee, if it has been paid. The examiner will inform the applicant that the application does not comply with the Patent Act and Patent Rules.
Date modified: January 2025
The Commissioner will withdraw a conditional notice of allowance before a patent is issued under subsection 86(14.1) of the Patent Rules if the examiner has reasonable grounds to believe that the application does not comply with the Patent Act or the Patent Rules, except when the defects would not affect the readability, clarity or validity of the patent, if it were granted. The conditional notice of allowance will not be withdrawn if the application is deemed abandoned.
An application may be withdrawn from conditional allowance under subsection 86(14.1) of the Patent Rules, for example, in view of applicable prior art identified in a protest or in a filing of prior art under section 34.1 of the Patent Act.
The Commissioner will withdraw a conditional notice of allowance under subsection 86(15) of the Patent Rules before a patent is issued if the applicant submitted a reply to the conditional notice of allowance in good faith within the time limit to respond, but after assessing the reply, the examiner believes the reply to be non-compliant with certain aspects of the Act and Rules. A reply is considered non-compliant if the defects identified in the conditional notice of allowance are not overcome by arguments or amendment. A reply is also considered non-compliant if it introduces impermissible amendments that contravene subsection 100(1) of the Patent Rules (e.g. amendments that are non-obvious and which do not address defects identified in the conditional notice of allowance).
If the reply is non-compliant, the Commissioner will inform the applicant by notice that the conditional notice of allowance has been withdrawn and refund the final fee, if it has been paid.
After a conditional notice of allowance is withdrawn under subsection 86(15) of the Patent Rules, the applicant will be informed of the outstanding defects in the application in an examiner’s report.
If a conditional notice of allowance is withdrawn by the Commissioner, any amendments made to the application after the conditional notice of allowance is sent and before the day on which the conditional notice of allowance is withdrawn are considered never to have been made.
Date modified: October 2022
Once the applicant pays the final fee, and, if applicable, corrects any minor defects noted in a conditional notice of allowance, the Office will prepare the application to issue a granted patent. If the payment is conditional, such as upon recordal of a transfer under section 49 of the Patent Act or upon amendment of an obvious error under subsection 100(2) of the Patent Rules, the Office will process those requests before starting the preparations to grant the patent, thus the currently recognized applicant or patentee will always be listed. Please consult CIPO’s service standards for information on the expected time to prepare a granted patent.
Date modified: December 2013
This chapter addresses examination practice surrounding the rejection of an application by an examiner, the writing of a Final Action to inform the applicant of the rejection, and the review of a rejected application by the Patent Appeal Board and the Commissioner of Patents.
Where an examiner, after having previously identified one or more defects in an application and having requisitioned the applicant to amend the application in order to comply with the Patent Act and Patent Rules or to provide arguments as to why it does comply, has considered the applicant’s response and has reasonable grounds to believe that the application still does not comply with the Patent Act or Patent Rules and that the applicant will not amend the application to comply, the application may be rejected.
A Final Action is the examiner’s report that notifies the applicant that their application has been rejected and that sets forth the examiner’s reasons for the rejection. In essence, rejecting an application and writing a Final Action is a mechanism that resolves impasses between an examiner and an applicant.
This chapter provides guidance on determining when a Final Action is warranted, the content of the Final Action itself, and the various post-rejection practices that lead to disposal of the application by allowance or refusal. A significant portion of the chapter details the practices of the Patent Appeal Board during the review of a rejected application by the Commissioner of Patents.
Date modified: October 2019
As is discussed in Chapter 12 of this manual, the examination of a patent application involves its consideration by a patent examiner.
Where, after examining the application, the examiner has reasonable grounds to believe that it complies with the Patent Act and Patent Rules, the examiner will approve the application for allowance [see section 25.01 of this manual].
Where, instead, the examiner considers that the application does not comply with the Patent Act or Patent Rules, the examiner will, in accordance with subsection 86(2) of the Patent Rules, inform the applicant of the application’s defects and requisition the applicant to amend the application to comply or to provide arguments as to why it does [see section 12.04 of this manual].
Examination typically proceeds through an exchange of examiner's reports and responses from the applicant. The aim of this process is to reach a conclusion as to the allowability of the application.
In some cases, the examiner and applicant will reach an impasse as to whether an identified defect truly is a defect. Where this occurs, the examiner will reject the application and notify the applicant in a Final Action.
Subsection 86(3) of the Patent Rules provides that:
If an applicant replies in good faith to the requisition made under subsection (2), on or before the date set out in subsection (4), but the examiner, after receiving the reply, has reasonable grounds to believe that the application for a patent still does not comply with the Act or these Rules in respect of any of the defects referred to in the requisition and that the applicant will not amend the application to comply with the Act and these Rules, the examiner may reject the application.
As will be seen later in the chapter, an applicant’s ability to amend the application after it has been rejected may be limited. Consequently, although an application can, in principle, be rejected as soon as an impasse occurs with respect to a single defect, in practice a rejection will usually not occur if the examiner considers that continued correspondence with the applicant is serving to resolve other substantive defects.
Broadly speaking, it is desirable for a Final Action to be written when all defects have been resolved other than those on which an impasse exists. In practice, where this would unduly prolong prosecution, a Final Action can be written even though an impasse has not been reached with respect to some defects. Furthermore, where an impasse has been reached on all the substantive issues previously identified as defects, but new defects (substantive or otherwise) were introduced by the applicant, these new defects can be identified in a Final Action.
The decision as to when it is appropriate to reject an application must be made considering the overall context of examination, including the length of prior prosecution, the nature of the outstanding defects remaining, the extent to which these had been discussed by the examiner and the applicant, and whether the examiner considers it likely that further prosecution would advance the application to allowance.
Subsequent to a rejection, the examiner will review any responses to the Final Action that were made by the applicant before the expiry of the time to respond. Where the examiner does not withdraw the rejection, the Patent Appeal Board and the Commissioner of Patents will review the rejected application, possibly in light of further submissions by the applicant. Following the review, the Commissioner may allow or refuse the application, or indicate a period of time during which the application may be amended in a manner specified by the Commissioner, such that it would be allowable if so amended but will otherwise be refused.
When an application has been refused by the Commissioner, the applicant may appeal the Commissioner’s decision to the Federal Court.
Date modified: October 2019
At each stage of examination, an examiner will endeavour to identify all the defects in the application and inform the applicant of these in a report in accordance with subsection 86(2) of the Patent Rules [see Chapter 12 of this manual].
Early in prosecution, it is possible that certain defects are interrelated, complicating their identification and resolution. Ambiguity in a claim, for example, could make it difficult to conclusively determine whether the claimed matter is novel or unobvious. As prosecution advances, the applicant’s amendments and arguments in response to a requisition may serve to change the examiner’s understanding of the invention. It is, thus, understandable that different or additional defects may be identified in subsequent reports.
It is also possible that an examiner may miss a defect during the analysis of the application; nevertheless it is required that the examiner identify these defects once aware of them.
As prosecution advances, it may become apparent that the examiner and applicant do not agree as to whether certain defects are present. Typically, where an applicant responds to a requisition by providing arguments as to why the application does comply but the examiner still considers that the application is defective, a further report identifying this same defect will provide a greater level of detail regarding the examiner’s analysis. As appropriate, the applicant’s arguments will be addressed in the examiner’s subsequent report.
Where it appears that prosecution is approaching an impasse, an examiner will usually advise the applicant of this fact by indicating in the report being written that a further report on substantially the same points may be made final. Although it is not a requirement of the Patent Act or Patent Rules that such a warning be provided, it should be done whenever doing so would be reasonable in the circumstances.[348]
The last report written before a Final Action (informally referred to as a “pre-final” action) should provide completely elaborated arguments supporting the examiner’s conclusion that the application is defective. Recognising that the applicant’s opportunities to amend the application subsequent to the expiry of the time to respond to a Final Action may be limited under subsection 86(7) of the Patent Rules, it is very important to ensure that all defects have been identified in a “pre-final” action. The limitations on amending the application post-rejection provide the reason for advising the applicant that the examiner is considering making the next report a Final Action: knowing their application faces imminent rejection, the applicant may consequently wish to take special care in responding to the pre-final action.
Date modified: October 2019
An examiner may reject an application where the requirements of subsection 86(3) of the Patent Rules are met, namely
Having rejected the application, the examiner then notifies the applicant of the reasons for having rejected the application under the provisions of subsection 86(5) of the Patent Rules, namely
If an examiner rejects an application for a patent, the examiner must send a notice bearing the notation “Final Action” or “Décision finale”, indicating the outstanding defects and requisitioning the applicant to amend the application in order to comply with the Act and these Rules or to submit arguments as to why the application does comply, not later than four months after the date of the notice.
Considering the guidance in section 26.03, it can be understood that the analysis of the defects identified in a Final Action is to be comprehensive in nature. Identifying a defect for the first time in a Final Action, while occasionally necessary, is generally not desirable. In particular, if a major defect (anticipation, obviousness, lack of utility, non-statutory subject-matter, insufficiency, etc.) was overlooked in prior prosecution, a further regular requisition identifying the defect is most likely necessary in order to allow the applicant an opportunity to have their response be evaluated prior to any rejection.
If a new, significant defect was introduced with amendments made in response to the previous report, the examiner will have to exercise judgement as to whether or not a Final Action is appropriate.[349]
While reasonable efforts must be made to avoid identifying a defect for the first time in a Final Action, it is also necessary to consider the effect of unduly prolonging prosecution. Where a new defect is introduced by the applicant late in prosecution, it may not be appropriate to delay rejection simply to deal with it. Furthermore, where a newly identified defect is readily understandable and easily fixed (e.g. a missing antecedent, incorrect claim numbering, etc.), it may not be necessary to delay rejection.
What should not be done, however, is to ignore an identified defect in order to simplify the Final Action. The examiner must decide whether a newly identified defect requires a further report under subsection 86(2) of the Patent Rules or if it can be included in a Final Action.
It should be noted that subsection 199(1) of the Patent Rules allows an examiner who has identified defects under subsection 30(2) of the Patent Rules then in force and has reasonable grounds to believe that the applicant will not amend the application to comply with the Patent Act and Rules, to reject the application.
Date modified: October 2019
A Final Action is a particular type of examiner’s report, and will usually not follow the regular style and form of a report written under subsection 86(2) of the Patent Rules.
The opening paragraph of a Final Action will identify that it contains a requisition under subsection 86(5) of the Patent Rules, and will feature the words FINAL ACTION prominently. The report will also include an indication that the application is being rejected pursuant to subsection 86(3) of the Patent Rules (or subsection 199(1) of the Patent Rules, where applicable).
The preamble of the report should identify, in broad terms, the defects that have led to the rejection and which claims are considered defective and which are allowable.
The entire report should be drafted bearing in mind the point of dispute. Where the examiner and the applicant agree on certain facts or conclusions pertaining to the disputed defect, this should be noted (with reference to any relevant correspondence) but it is not necessary to comprehensively revisit these aspects.[350]
The goal of the Final Action is to make the point of disagreement clear, to set out the applicant’s position as understood by the examiner, and the examiner’s reasoning for considering the application to still not comply with the Act or Rules. The Final Action should be drafted so that interested persons reading it (including the applicant, Patent Appeal Board, the Commissioner or the Court) can readily understand the point of the dispute and the examiner’s reasons for concluding that the application does not comply with the Act or Rules despite any arguments to the contrary from the applicant.
Although the actual layout and presentation of a Final Action can be tailored to fit the facts of the case under consideration, the following information should be provided where relevant.
It may be beneficial to divide the report into sections, using clear headings to identify what is being discussed in each section.
To the extent practical, the Final Action should be written so that it can be understood independently of other reports or responses. More particularly, pertinent arguments should not be incorporated by reference to other documents but should, minimally, be summarised in the Final Action itself.
Date modified: December 2013
An applicant may respond to a Final Action by submitting amendments to make the application compliant with the Patent Act and Patent Rules or by submitting arguments as to why the application does comply.
Upon receipt of a response to the Final Action before the expiry of the time to respond, the examiner will review the application.
Date modified: September 2020
If, after considering any amendments and arguments submitted by the applicant, the examiner considers that the application complies with the Act and Rules, it will be allowed pursuant to subsection 86(6) of the Patent Rules, which provides that
If an applicant, on or before the date set out in subsection (8), replies in good faith to a requisition made under subsection (5) and the examiner has reasonable grounds to believe that the application for a patent complies with the Act and these Rules, the Commissioner must by notice inform the applicant that the rejection is withdrawn and the application has been found to be allowable and require the payment of the final fee (see CIPO’s website on Patent Fees) not later than four months after the date of the notice.
For the purpose of applications subject to a Final Action under subsection 30(4) of the Patent Rules then in force, it should be noted that if an examiner determines that after a response to a Final Action the application does comply with the Patent Act and Rules in force, the Commissioner sends a notice to inform the applicant that the rejection is withdrawn, the application will be allowed and requires payment of the final fee as per subsection 199(2) of the Patent Rules.
Date modified: October 2019
If, after considering any amendments and arguments submitted by the applicant, the examiner considers that the application still does not comply with the Act or Rules, the examiner’s next steps depend on whether the time to respond to the requisition has expired or not.
If the time to respond has not expired, the examiner may contact the applicant to inform them of the examiner’s conclusions and to determine whether the applicant wishes to submit further amendments and/or arguments prior to the expiry of the time to respond to the requisition. This would be particularly appropriate in instances where the applicant has partially addressed the grounds for rejection and where it appears a further response could make the application allowable.
If the time to respond to the requisition has expired, the provisions of subsection 86(7) of the Patent Rules apply. Thus,
If an applicant replies in good faith to a requisition made under subsection (5) on or before the date set out in subsection (8) but, after that date, the examiner still has reasonable grounds to believe that the application for a patent does not comply with the Act or these Rules,
(a) the Commissioner must by notice inform the applicant that the rejection has not been withdrawn;
(b) any amendments made to that application during the period beginning on the date of the final action notice and ending on the date set out in subsection (8) are deemed not to have been made; and
(c) the application must be reviewed by the Commissioner.
By virtue of paragraph 86(7)(b) of the Patent Rules, any amendments made after the Final Action was sent are considered not to have been made unless the examiner determines that they place the application in condition for allowance. If, after the time for responding to the Final Action has expired, an examiner concludes that the application is still not allowable, the examiner will prepare the case for review by the Commissioner.
For the purpose of applications subject to a Final Action under subsection 30(4) of the Patent Rules then in force, it should be noted that if an examiner believes that the application does not comply with the Act or the Rules in force, a notice will be sent as per subsection 199(3) of the Patent Rules informing the applicant that the rejection has not been withdrawn, any amendments will be deemed not to have been made and the Commissioner will review the application in accord with subsections 86(9)-86(13) of the Patent Rules.
Date modified: October 2019
A Summary of Reasons is a document written by an examiner in preparation for the Commissioner’s review of a rejected application pursuant to paragraph 86(7)(c) of the Patent Rules. It is written only when the time to respond to the Final Action requisition has expired and the applicant’s response has not overcome the reasons for rejection [see 26.05.02].
In the Summary of Reasons, the examiner briefly sets out why they still do not have reasonable grounds to believe that the application complies with the Patent Act and Patent Rules. Since the rejection is being maintained, any amendments proposed by the applicant subsequent to the rejection are considered not to have been made.
Consequently, the examiner’s reasons for considering the application not to comply with the Act and Rules will primarily be those set out in the Final Action itself. Reasons given in the Final Action should not be comprehensively repeated in the Summary of Reasons, which (as its name implies) is intended to be a brief document.
The Summary of Reasons should identify and address any new considerations arising from the applicant’s post-rejection correspondence received up to the expiry of the time to respond to the requisition, such as new arguments in support of patentability, relevant jurisprudence or changes to Office practice.
If the applicant has proposed amendments, the examiner should provide a concise analysis of the effect of these amendments. The Summary of Reasons will provide information such as whether proposed amendments would have overcome, or addressed in part, certain of the examiner’s grounds for considering the application defective or would have changed the examiner’s reasons for considering the claims defective. It would be particularly noted if the proposed amendments would have rendered certain claims allowable. Similarly, any defects present in the proposed amendments would be identified.
It should also be indicated if certain of the applicant’s arguments were compelling, even if the arguments themselves were insufficient to give the examiner reasonable grounds to consider the application to comply with the Act and Rules. This might be the case, for example, where an applicant explains how the invention may be distinguished from cited prior art, but the arguments are based on features not defined in the claims.
In view of the above, it can be understood that the Summary of Reasons is intended to assist in the review of the application by providing a concise, high-level overview of important considerations arising from any post-rejection correspondence with the applicant as well as any information relevant to the review which was not available at the time the application was rejected.
Date modified: October 2019
A review of a rejected application is, as previously noted, required by subsection 86(7) of the Patent Rules whenever the applicant’s response to a Final Action does not place the application in condition for allowance.
While the review is primarily focused on resolving the impasse that led to a rejection, the review is also comprehensive, meaning that any apparent defects in the application, even beyond those indicated in the Final Action and/or the Summary of Reasons, will be identified at this stage.[351] This point is highlighted in paragraph 86(7)(c) of the Patent Rules which states that the application is reviewed.
It can be broadly stated that the intention of the review process is to achieve efficiency, finality, and compliance of the application with the Patent Act and Patent Rules while adhering to the principles of natural justice and procedural fairness.
The review of an application can be terminated by withdrawing the application, and will typically not proceed during periods where the application is deemed abandoned by operation of law. The review is also terminated where an application remains abandoned outside the reinstatement period.
Date modified: September 2017
The Commissioner is assisted in performing the review of a rejected application by the Patent Appeal Board (PAB).[352] The PAB is an advisory body consisting of a Chair and several members, each of whom is a senior official of the Patent Office with previous experience as a patent examiner. The review of a specific application is typically performed on behalf of the Commissioner by a panel of three members of the PAB. In order that the review of the application be impartial, these members must not have participated in the prosecution of the application or have previously given advice in respect thereto.
The review occurs only after the time limit for responding to the Final Action has expired and the Summary of Reasons has been prepared and forwarded to the PAB. At this point, control over the application is transferred to the PAB.
It is to be noted that the review process is an ex parte process, meaning that there is only one party to the proceedings, namely the patent applicant. The process is a continuation of the administrative procedures of the office with regard to patent applications under the Patent Act, but is performed at arm’s length to the examination divisions.
Date modified: October 2019
During the review process, an applicant can expect to be contacted by the Board several times. These communications may cover both administrative and substantive matters relating to the review.
Administrative matters include informing the applicant that the application has been transferred to the PAB and details relating to giving the applicant an opportunity to be heard.
Substantive matters include keeping the applicant informed of any matters affecting the review, including providing the applicant with a copy of the Summary of Reasons.
When a rejected application is transferred to the PAB, the applicant is informed in an initial letter from the Board. This initial letter will, minimally, notify the applicant, as required by paragraph 86(7)(a) of the Patent Rules (or paragraph 199(3)(a) of the Patent Rules, where applicable), that the examiner’s rejection has not been withdrawn [see 26.05.02] and that the case has been transferred to the PAB. A copy of the Summary of Reasons [see 26.06] will accompany the letter.
Where the applicant responded to the Final Action by submitting amendments, the initial letter will also confirm, per paragraph 86(7)(b) of the Patent Rules (or paragraph 199(3)(b) of the Patent Rules, where applicable), that because the rejection was not withdrawn, any amendments received in response to the Final Action within the time referred to in subsection 86(5) are considered not to have been made.
Additional information relating to the review, including the offer of an opportunity to be heard, may be included in the initial letter or dealt with separately.
Communications from the PAB generally include a time period to respond. It is important to note, however, that a letter from the PAB is not a requisition. If it is not responded to within the time period stated, the application will not be deemed abandoned. Consequently, failure to respond to a PAB communication will not suspend the review process.
Date modified: October 2019
During the review, the panel may come to believe that defects beyond those identified in the Final Action are present in the application. The identification of such defects may result, for example, from the panel interpreting the application differently from the examiner, or be in view of different interpretations of jurisprudence or office practices, or be in view of new art submitted through a late-filed protest, art cited in recent foreign prosecution or a change in the Patent Act or Patent Rules.
Where a new defect is identified during the review, the applicant is given notice of the issue and an opportunity to respond, which includes the possibility of proposing amendments to address the defect. Amendments proposed by the applicant, if they correct the defect, may be later required to be made by the Commissioner in a Commissioner’s Decision under paragraph 101(b) of the Patent Rules [see 26.08.03]. The opportunity to respond is demanded both by the requirements of natural justice and by subsection 86(9) of the Patent Rules, which provides that:
If, during the review of a rejected application for a patent, the Commissioner has reasonable grounds to believe that the application does not comply with the Act or these Rules in respect of defects other than those indicated in the final action notice, the Commissioner must by notice inform the applicant of those defects and invite the applicant to submit arguments, not later than one month after the date of the notice, as to why the application does comply.
Where a potential defect is identified during the review process, the panel may raise the matter directly with the applicant or may request that the examiner provide an analysis in relation thereto. In exceptional cases, the panel may also determine that a further search and analysis of the prior art is necessary in relation to the defect.
Where an analysis is requested of an examiner, the examiner’s findings are presented in a Supplemental Analysis, a document similar in form to a Summary of Reasons but addressing only the issue identified by the panel.
Where a Supplemental Analysis is requested of an examiner, the applicant will be duly informed and will receive a copy of the analysis.
A response to a Supplemental Analysis, including proposed amendments, should only address the defect under consideration in the analysis.
Date modified: September 2017
It is desirable that the review proceed, as far as is reasonably practical, on the basis of a common understanding of the matters at issue. Therefore, in addition to the identification of new defects, it is also possible that the panel may wish to clarify certain other matters with the applicant during the review process.
Such clarifications are intended to ensure that the applicant and the panel have the same understanding of, for example, the examiner’s grounds for rejection, the applicant’s arguments, the applicable Office practice, or of certain relevant facts.
Where it appears to the panel that clarification is desirable, a memo will be sent to the applicant setting out the matters that, in the panel’s view, may require clarification. Where the examiner’s input is necessary, it may be provided in the form of a Supplemental Analysis.
The applicant will be given a period of time to respond, and may respond with written submissions or with oral arguments at the hearing.
Date modified: October 2019
Subsection 86(13) of the Patent Rules specifies that the applicant must be given an opportunity to be heard before any refusal. The applicant will therefore generally be invited to participate in a hearing. The PAB will make reasonable efforts to accommodate the applicant’s schedule, but if the applicant is unable to participate in a timely hearing the review will proceed nonetheless.
The applicant is not required to attend a hearing, and may instead request that the review proceed on the basis of the written record.
Prior to any hearing, the panel will perform an initial review of the case both to ensure that the outstanding issues have been clearly identified and articulated and that there are no other issues requiring clarification, such as defects identified pursuant to subsection 86(9) of the Patent Rules [see 26.07.03].
The purpose of the hearing is to provide the applicant with a further opportunity to develop and explain the reasons for contending that the application is not defective (on the basis of the grounds raised either by the examiner or by the PAB during the review process) or that proposed amendments overcome the identified defects. Written arguments and/or additional evidence should be presented to the panel well ahead of the hearing, to ensure the panel has sufficient time to consider them. When any new legal or technical argument or fresh evidence relevant to the grounds raised by the examiner comes to the applicant’s attention it should be presented as early as possible and not deferred until the review stage (i.e., the applicant’s best case should be made during prosecution before the examiner and not only before the PAB).
The hearing may occur in person, via teleconference or via videoconference, at the option of the applicant, and may include the assigned panel, the applicant and applicant’s patent agent and/or associate patent agent, as well as the examiner and the examiner’s supervisor.
The hearing is primarily an opportunity for the applicant to present its position in order to advance prosecution, with input from the panel.
Typically a hearing begins with an oral presentation by the applicant. The panel may pose questions to the applicant during or after the applicant’s presentation of arguments, depending both on the need to intervene and the applicant’s preferences. The examiner and examiner’s supervisor are normally present and may be called upon by the panel to answer questions in relation to the defect(s) and any technical matters. The applicant is given an opportunity to make any final comments before the conclusion of the hearing. No cross-questioning between the applicant and examiner is permitted.
Points of fact agreed to during the hearing, or concessions made by the applicant, will be taken into account in the recommendation to the Commissioner. Although it is expected that the applicant will be prepared to address any questions posed at the hearing, it may be acceptable, should an unexpected issue arise during discussion, for the applicant to make additional submissions to the panel within a reasonable period thereafter.
Since the panel must make a recommendation to the Commissioner, no decision regarding disposal of the application may be made at the hearing.
Date modified: October 2019
It is not necessary in every case to hold a hearing. As noted in 26.07.04, the applicant may decline the invitation for a hearing. Where this is done, the assigned panel will review the case and provide a recommendation to the Commissioner taking into account the written record before it, including any further written submissions the applicant has provided.
It is also possible that the panel, after its initial review of the case, may conclude that the application complies with the Act and Rules. Where the Commissioner agrees with this conclusion, there is no need to invite the applicant to attend a hearing. Subsection 86(13) of the Patent Rules does not require a hearing where the application will be allowed.
Date modified: September 2017
At the conclusion of the panel’s review, the panel will deliberate and formulate a recommendation to the Commissioner. The panel considers the facts and law related to the particular matter before them, including any arguments and evidence adduced by the applicant during the review.
The recommendation is provided as written reasons that generally include an explanation of the invention being considered, background information on the prosecution, an identification of the issues to be decided, relevant statutory authority, pertinent jurisprudence, a summary of the positions of the examiner and applicant, a detailed analysis of the issue(s) including factual findings, and a final recommendation of the panel.
The Commissioner of Patents is then briefed on the case and reviews the recommendation prior to rendering a final decision.
Date modified: June 2016
The Commissioner’s Decision provides reasons for arriving at the decision and explains any findings with reference to the Patent Act, Patent Rules and pertinent jurisprudence. Typically, the Commissioner adopts the panel’s reasons.
In addition to its importance to the applicant, a Commissioner’s Decision can also provide insight and/or guidance to applicants and patent examiners as to the current understanding of the state of the law and Office practice. Commissioner’s Decisions are carefully reviewed when practice guidance is provided to examiners.
A copy of the decision is sent to the applicant (by registered mail if the application is refused, as per section 40 of the Patent Act). These decisions become part of the prosecution file and are therefore open to public inspection, except for decisions made in respect of applications filed prior to October 1, 1989 which are only published with the permission of the applicant.
A database of published Commissioner’s Decisions is maintained by the Office and may be accessed via the CIPO web site.
In the following sections, the possible outcomes of Commissioner’s Decisions are set out, along with the effect of each.
Date modified: September 2020
Subsection 86(10) of the Patent Rules provides that
If, after review of a rejected application for a patent, the Commissioner has reasonable grounds to believe that the application complies with the Act and these Rules, the Commissioner must by notice inform the applicant that the rejection is withdrawn and that the application has been found allowable and require the payment of the final fee (see CIPO’s website on Patent Fees) not later than four months after the date of the notice.
In such a case, the applicant will be notified in the Commissioner’s Decision that the rejection is withdrawn and that the application will be allowed.
Once the application has been allowed, it is treated in the same manner as any other allowed application [see section 25.01 of this manual], with a notice of allowance being sent to the applicant requisitioning payment of the final fee.
Date modified: October 2019
If upon review of the rejected application the Commissioner is of the view that the examiner’s rejection is justified, or that the application does not comply with the Act or Rules on the basis of defects identified during the review process, and it is not evident that the application can be made compliant through a directed amendment per paragraph 101(b) of the Patent Rules, the Commissioner will refuse the application pursuant to section 40 of the Patent Act. The refusal will be indicated in the Commissioner’s Decision which will also specify the applicable six month period in which to initiate an appeal to the Federal Court.
Date modified: October 2019
As per subsection 86(11) of the Patent Rules
If, after review of a rejected application for a patent, the Commissioner has reasonable grounds to believe that the application does not comply with the Act or these Rules and certain amendments are necessary in order to make the application allowable, the Commissioner must by notice inform the applicant that those amendments must be made within three months after the date of the notice.
The applicant will be notified of the necessary amendments in the Commissioner’s Decision pursuant to subsection 86(11) of the Patent Rules and will be invited to make the amendments pursuant to paragraph 101(b) of the Patent Rules. The amendments required in a Commissioner's Decision may be based on proposed amendments submitted during the review process, both as a result of the applicant's own initiative or as a result of defects identified during the review process. They may also be based on the Commissioner’s findings alone as to how the application can be made compliant with the Act and Rules.
If in response to the requirement for amendment the applicant does not make the necessary amendments, or makes amendments beyond those required, the Commissioner will refuse the application in accordance with section 40 of the Patent Act.[353]
For the purpose of applications for which the Commissioner invited amendments by way of a notice under subsection 30(6.3) of the former Patent Rules, it should be noted that if an applicant has complied with the notice, the Commissioner will send a notice informing the applicant that the rejection is withdrawn, that the application has been found allowable and require payment of the final fee not later than four months after the date of that notice as per subsection 199(5) of the Patent Rules.
Date modified: December 2013
Where the Commissioner refuses a patent application under section 40 of the Patent Act, section 41 of the Act states that
Every person who has failed to obtain a patent by reason of a refusal of the Commissioner to grant it may, at any time within six months after notice as provided for in section 40 has been mailed, appeal from the decision of the Commissioner to the Federal Court and that Court has exclusive jurisdiction to hear and determine the appeal.
The decision of the Federal Court may be appealed to the Federal Court of Appeal and, with leave, to the Supreme Court of Canada.
Date modified: October 2019
Following a decision of the Court, the Commissioner takes action in accordance with any resulting orders of the Court. Of note is that the Court has the authority to order the entering of amendments, per paragraph 101(c) of the Patent Rules
if an application for a patent is rejected by an examiner under subsection 86(3), the specification and drawings contained in the application must not be amended by the applicant after the date prescribed by subsection 86(8), except if (c) the Supreme Court of Canada, the Federal Court of Appeal or the Federal Court orders the amendments to be made.
For the purpose of applications rejected under subsection 199(1) of the Patent Rules or subsection 30(3) of the former Patent Rules, it should be noted that as per section 200 of the Patent Rules, if an application is rejected by an examiner, the specification and drawings contained in the application must not be amended after the date prescribed by subsection 199(4) of the Patent Rules, except if
(c) the Supreme Court of Canada, the Federal Court of Appeal or the Federal Court orders the amendments to be made.
Date modified: December 2025
A patentee who holds the rights to a patent in Canada must pay annual maintenance fees starting at the 2nd anniversary of the filing date to maintain the patent in effect according to subsection 46(1) of the Patent Act and subsection 112(1) of the Patent Rules.
If an additional term is granted under section 46.1 of the Patent Act, the patentee must pay maintenance fees starting on the 20th anniversary of the filing date (subsection 46.2(1) of the Patent Act and section 117.05(1) of the Patent Rules).
Please see Chapter 5 for information on who can pay maintenance fees and late fees for patents.
Date modified: December 2025
The amounts and time limits for paying maintenance fees to maintain a patent in effect can be found on CIPO’s webpage on Patent Fees. Maintenance fees are due annually on or before the anniversary of the filing date, starting on the 2nd anniversary of the filing date.
Any or all of the maintenance fees for a particular patent may be paid in advance. In accordance with subsection 112(4) and subsection 117.05(2) of the Patent Rules, the time limits for payment of maintenance fees for patents cannot be extended.
Date modified: December 2025
If a patent is granted on the basis of a patent application for which a maintenance fee for an anniversary of the filing date that fell within the 12-month period was not paid before the day on which the patent was issued, the amount of that unpaid maintenance fee will be added to the first maintenance fee after the patent is granted. A late fee will also be required (subsection 112(5) and subsection 117.05(3) of the Patent Rules).
Date modified: December 2025
If the full required maintenance fee is not paid on or before the anniversary date, a late fee will also need to be paid (paragraph 46(2)(a) of the Patent Act, section 115 and section 117.08 of the Patent Rules). A Commissioner’s Notice under paragraph 46(2)(b) or paragraph 46.2(1)(b) of the Patent Act, as applicable, will be sent to the patentee shortly after the maintenance fee due date. The notice will require the patentee to pay the maintenance fee and the late fee before the later of:
The period between the original maintenance fee due date and the later of six months from the due date or two months from the date of the notice is the late fee period.
Date modified: December 2025
If the maintenance fee and the late fee are not paid within the late fee period, the term of the patent will be deemed to have expired retroactively at the original maintenance fee due date under subsection 46(4) of the Patent Act and section 113 of the Patent Rules.
If the maintenance fee and the late fee are not paid within the late fee period for a patent for which additional term is granted, the additional term of the patent will be deemed to have expired retroactively at the original maintenance fee due date under subsection 46.2(4) of the Patent Act and section 117.06 of the Patent Rules.
Date modified: October 2019
While not required by the Patent Act or the Patent Rules, the Patent Office will endeavour to inform patentees of deemed expiry through a courtesy letter. Please note that in all cases, patentees will have received a notice of the potential for deemed expiry if they didn’t comply with the requirements to pay the maintenance fee and the late fee.
Date modified: December 2025
If a patent or an additional term is deemed to have expired under subsection 46(4) or 46.2(4) of the Patent Act, it is possible, under subsection 46(5) or 46.2(5), as applicable, of the Patent Act and sections 116 , 117, or sections 117.09 and 117.1, as applicable of the Patent Rules, to make a request to the Commissioner to reverse the deemed expiry.
The deemed expiry will be reversed only if the Commissioner makes a determination that the failure occurred in spite of the due care required by the circumstances was taken (paragraph 46(5)(b) or paragraph 46.2(5)(b), as applicable of the Patent Act).
Date modified: December 2025
Under section 116 and 117.09(1) of the Patent Rules, the time limit for a patentee to request the reversal of a deemed expiry of a patent or of an additional term is 12 months after the end of six months after the applicable maintenance fee due date. Both the six-month and the 12-month periods will be extended under section 78 of the Patent Act if they expire on a prescribed day or a designated day. An example is listed below for illustrative purposes:
Example:
The maintenance fee for a patent is due on March 31 and it is not paid by the due date. The Commissioner’s Notice is sent on April 15 requiring the patentee to pay the fee and late fee before the later of 2 months from the date of the notice or 6 months from the maintenance fee due date. The later date is 6 months from the maintenance fee due date or September 30 of the same year. The maintenance fee and the late fee are not paid by September 30 therefore the patent is deemed expired retroactively at the maintenance fee due date of March 31. The period in which to request the reversal of the deemed expiry ends on September 30 of the next year.
Date modified: December 2025
Amendments made to the Patent Act and the Patent Rules (SOR/2019-251) to implement the Patent Law Treaty (PLT) introduce a due care standard that must be met by a patentee if they wish to apply for reversal of deemed expiry of a patent or of an additional term because of a failure to pay a prescribed maintenance fee and the late fee.
Subject to the transitional provisions below, the Commissioner is required to make a determination that a failure occurred – in spite of the due care required by the circumstances having been taken – before the patent or the additional term can be deemed never to have expired under subsection 46(5) or subsection 46.2(5) of the Patent Act.
Date modified: October 2022
Section 78.55 of the Patent Act specifies that section 46 of the Patent Act, as it read immediately before October 30, 2019, applies to maintenance fee due dates (not including the period of grace) before October 30, 2019. If the maintenance fee due date (not including the period of grace) is before October 30, 2019 and the maintenance fee, which can be found on CIPO’s webpage on Patent Fees, is not paid on or before that due date, the patentee will have a 12-month grace period.
Date modified: December 2025
In order for the Commissioner of Patents to make a determination, the patentee is required to provide the reasons for the failure to take the action that should have been taken to avoid the deemed expiry of the patent or the additional term. If the Commissioner makes a determination that the failure occurred in spite of due care having been taken by the patentee, the deemed expiry under subsection 46(4) or subsection 46.2(4) of the Patent Act is then deemed to never have occurred. The deemed expiry of a patent or of an additional term will be reversed only if the applicable requirements set out in subsection 46(5) or subsection 46.2(5) of the Patent Act are met and the Commissioner informs the patentee of this determination.
Date modified: October 2019
When determining whether the failure occurred in spite of the required due care having been taken by the patentee, the Commissioner will assess whether the patentee took all measures that a reasonably prudent patent holder would have taken – given the set of circumstances related to the failure – to avoid the failure, and for the failure to have occurred despite having taken those measures. Measures taken by the patentee after the failure occurred will not be taken into consideration in making the determination. This approach is generally consistent with the approach that is currently used by CIPO when acting as a Receiving Office in the context of a request for restoration of priority under the Patent Cooperation Treaty, when that request for restoration of the right of priority is made on the basis that due care required by the circumstances was taken.
Date modified: December 2025
The patentee must, within twelve months after the end of six months after the applicable maintenance fee due date meet the following requirements set out in paragraph 46(5)(a) or paragraph 46.2(5)(a) of the Patent Act to reverse the deemed expiry of the patent or of an additional term:
Date modified: October 2019
In order to make a determination of whether the failure occurred in spite of due care required by the circumstances having been taken, the Commissioner will consider the reasons for the failure to act that are provided by the patentee. In order to assist the Commissioner in making a determination, the Patent Office recommends that the patentee include, as part of the required reasons for the failure, the following elements in the request for reversal of deemed expiry:
The patentee may also include evidence of the circumstances and reasons for failure, such as a medical note or other relevant affidavits. For information on protecting your privacy, please see Section 8.02.05a in Chapter 8.
Date modified: October 2019
The Commissioner will review the reasons for the failure to pay the maintenance fee and the late fee to determine whether the failure occurred in spite of the due care required by the circumstances having been taken. In making a determination of whether the failure occurred in spite of the due care required under the circumstances having been taken, the Commissioner will consider whether anything else could have been reasonably expected to have been done to avoid the failure while taking into consideration the particular set of circumstances surrounding the failure to take the required action. Measures taken by the patentee after the failure occurred will not be taken into consideration in making the determination. In making this determination, the Commissioner will consider the customary diligence that a prudent party would have exercised in the circumstances.
In making this determination, the Patent Office will have regard to considerations that are taken into account by the International Bureau and Receiving Offices as described in paragraph 166M of the Receiving Office Guidelines, while acknowledging that no two cases with have identical sets of facts or circumstances.
In general, under the following circumstances, a determination that a failure occurred in spite of due care required under the circumstances having been taken by the patentee, the patent agent or other person authorized by the patentee may be made where those people demonstrate the due care of a reasonably prudent person that would be required by the circumstances was taken:
In general, the following circumstances may favour a determination that due care required by the circumstances was not taken by the patentee, the patent agent or other person authorized by the patentee :
Date modified: December 2025
Before any determination is made by the Commissioner under paragraph 46(5)(b) or paragraph 46.2(5)(b) of the Patent Act on whether the required due care under the circumstances was not taken, the Patent Office will send a letter to the patentee informing them of the Commissioner’s intended determination and provide the patentee with the opportunity to make observations before the end of one month after the date of the letter.
Date modified: October 2019
Unless the patentee is informed that the Commissioner intends to determine that due care required by the circumstances was not taken, they can expect a response to a request for a reversal of the deemed expiry determination, including the Commissioner’s determination with respect to the due care standard, within two months of receipt of the request in the Office, or two months from receipt of the last correspondence relating to the request.
Date modified: September 2020
From time to time, a patent can contain errors due to an oversight by the patentee or the Patent Office. For that reason, the Office encourages applicants to review all documents prior to submission as well as the specification and drawings on file in the Office when a notice of allowance is sent to ensure that they are error free. Certain errors in granted patents may be corrected.
Date modified: September 2020
The Commissioner may under subsection 107(1) of the Patent Rules correct errors, made by the Commissioner in the patent or in the specification or drawings referenced in the patent within 12 months after the issue of the patent or on request of the patentee made within that period. In order for the Commissioner to make the correction, it must be obvious based on the documents on file in the Patent Office that something else was intended and that nothing else could have been intended. No fee is required for the correction of obvious errors made by the Commissioner.
The Patent Office has a rigorous quality process to ensure that the patents it issues are free of errors though they do happen occasionally. Though the Commissioner can correct these errors without a request from the patentee, the Patent Office recommends that patentees review their granted patent upon receipt and signal any errors to the Commissioner immediately since the time to make these corrections is short.
All requests for corrections of Commissioner’s errors in a granted patent must comply with the requirements for submitting written communications to the Commissioner as described in Chapter 2. Every request must include:
Date modified: September 2020
Under section 108 of the Patent Rules, the Commissioner may, on his or her own initiative within six months after the day on which a certificate is issued under section 48.4 of the Patent Act, or on request of the patentee within that period, correct an error by the re-examination board in the certificate if, from the documents that were in the possession of the Patent Office on that day, it is obvious that something other than what is in the certificate was intended and that nothing other than the correction could have been intended.
Date modified: October 2022
Patentees are able, within 12 months of the patent’s issuance, to request correction of errors in the naming of the patentee or the inventor so long as it does not change their identity under paragraph 109(1)(a) of the Patent Rules. The correction may be made after the 12-month period so long as the request is submitted before the end of 12 months after the day the patent is issued. The request must contain a statement to the effect that the correction does not add or delete the name of a patentee or an inventor or change the identity of a named patentee or inventor, as the case may be.
Date modified: October 2019
Patentees are able, within 12 months of the patent’s issuance, to request correction of obvious errors in the specification or drawings under paragraph 109(1)(b) of the Patent Rules. The error must have been obvious to a person skilled in the art that something else was intended than what appears AND that nothing else could have been intended other than the correction proposed by the patentee in their request.
Date modified: September 2020
In accordance with subsection 109(2) of the Patent Rules, the request for correction of an error in a patent under subsection 109(1) of the Patent Rules must contain:
If the patentee makes a request to correct an error in a patent but does not comply with the requirements and/or does not pay the prescribed fee, the Commissioner will send a notice requiring the patentee to submit the required information and/or pay the prescribed fee within three months after the date of the notice. If the required information and/or fee are not received within that time, the request for correction will be considered never to have been made.
The grant copy of a patent should never be submitted to the Patent Office when requesting a correction.
The prescribed fee is required to make the correction request, and payment is not contingent upon the acceptance or refusal of the request for correction.
Date modified: September 2020
If the Commissioner corrects an error under sections 107, 108 or 109 of the Patent Rules, the Commissioner will issue a certificate setting out the correction. The correction has a retroactive effect and will be considered to have been made on the date on which the patent or certificate was issued.
Date modified: October 2019
Disclaimer is a mechanism whereby a patentee may, at any time during the life of a patent, amend a patent to claim less than that which was claimed in the original patent. It is used where the patentee has, “by any mistake, accident or inadvertence, and without any wilful intent to defraud or mislead the public”
, made a specification “too broad” by claiming more than the inventor invented or subject-matter to which the patentee had no lawful right[354] (subsection 48(1) of the Patent Act). A disclaimer is not necessarily limited to a whole claim or claims. A part of a claim may be disclaimed,[355] provided that the disclaimer does not extend the scope of the claim or any claims depending on the claim.[356]
A filing of a disclaimer is a renunciation of subject-matter. A disclaimer is also a clear and unequivocal statement that the original patent claims are too broad and thus invalid.[357]
Date modified: September 2020
To file a disclaimer, Form 2 of Schedule 1 of the Patent Rules must be completed and filed with the Patent Office along with the appropriate fee (see CIPO’s website on Patent Fees, subsection 48(2) of the Patent Act and section 120 of the Patent Rules). In completing Form 2, the patentee must follow the precise form of subsections 3(1) and 3(2), which specify the subject-matter disclaimed. The expression “...with the exception of the subject-matter of the invention defined by the following claim:” in Form 2, subsection 3(2) indicates the other claim(s) defining those elements of the partially-disclaimed claim(s) remaining after the disclaimer, and cannot be used to reformulate or redefine the invention disclosed and claimed.[358]
Date modified: October 2019
The filing of a disclaimer does not involve any examination of the subject-matter of the claims by the Patent Office. The Patent Office only ensures that the disclaimer has been filed in the prescribed form and manner in accordance with subsection 48(2) of the Patent Act and sections 120 and 121 of the Patent Rules. As long as the disclaimer is filed in the proper form and manner, and the prescribed fee has been paid, the Commissioner has no discretion to refuse to record it.[359]
The onus of showing that the disclaimer satisfies all the requirements of subsection 48(1) of the Patent Act rests with the patentee. Furthermore, there is no presumption of validity of the disclaimer.[360] The validity of the disclaimer depends on: the state of the mind of the patentee at the time of preparation of the specification;[361] whether the disclaimer is made in good faith and not for an improper purpose;[362] the length of time between discovering a problem with the patent and the filing of a disclaimer;[363] whether the disclaimer broadens the scope of the patent;[364] whether the disclaimer recasts an invention;[365] or, whether the disclaimer adds a new and different combination by the addition of elements to the claim.[366]
If the courts determine that the disclaimer is invalid, the disclaimed claims must return to how they were prior to the disclaimer. However, the disclaimed claims as they stood prior to the disclaimer are invalid[367] on the basis of being overbroad by admission of the patentee.[368]
Date modified: December 2015
Disclaimers do not normally affect any court action pending at the time they are made (subsection 48(4) of the Patent Act).
Following a disclaimer, the remaining claims are deemed to be valid for the matter not disclaimed, i.e. in their disclaimed form (subsection 48(6) of the Patent Act). Thus a claim which is overly broad which has not been adjudged to be invalid may be saved from a finding of invalidity if a valid disclaimer is filed but only if filed in a timely way.[369]
The disclaimer is unconditional. The existing claims of the patent are the claims as amended by virtue of the disclaimer, and the only invention protected by the letters patent is that defined by such existing claims.[370]
Date modified: December 2015
The purpose of re-examination is to provide a relatively summary and inexpensive alternative to an impeachment process by litigation or an opportunity for a patentee to have the Patent Office reconsider the claims of an issued patent.[371]
As noted by the Federal Court in Prenbec v Timberblade, re-examination proceedings are less comprehensive in nature than an impeachment action (para. 48). For example, unlike an action, a re-examination proceeding is limited to issues arising from the prior art supplied by the requesting party. Additionally, the re-examination board does not possess the means to test the credibility of contested issues of fact (para. 34) that are available to a Court, such as by hearing from live witnesses under cross-examination (para. 47).[372]
The re-examination process is set out in sections 48.1 to 48.5 of the Patent Act. Any person, including the patentee, may request re-examination of any claim or claims of a patent issued after October 1, 1989, at any time during the life of the patent, based on prior art. This applies to patent applications filed before October 1, 1989 which issued thereafter.
Upon receipt of an acceptable request for re-examination in accordance with subsections 48.1(1) and (2) of the Patent Act, re-examination proceeds in one or two stages, depending on the outcome of the first stage, both of which are ex parte in nature. If the requester is also the patentee they will participate in the second stage. In cases where the requester is not the patentee, any submissions from the requester beyond the filing of an acceptable request will not be acknowledged or taken into account during re-examination.
The first stage involves a preliminary decision by a re-examination board established by the Commissioner of Patents as to whether the request raises a substantial new question of patentability. The preliminary decision includes the re-examination board’s reasons as to why a substantial new question of patentability is or is not raised by the request.
Where a substantial new question of patentability has been raised, the second stage involves the re-examination of the patent based on this question.
In the re-examination process, the board is not an adverse party as would be a competitor in an impeachment proceeding. The board’s role is rather that of an adjudicator in an administrative context. The expertise of its members may be taken into account in any determinations which are made as part of the board’s statutory duties.[373] In making factual determinations based on the record before it, such as who is the ordinary person skilled in the art and what was the relevant common general knowledge, there is no burden on a re-examination board to seek out and locate independent evidence to support these conclusions.[374] Such determinations are considered by a reviewing court based on a reasonableness standard and taking into account the board’s expertise.[375]
The Patent Appeal Board is tasked with the administration of the re-examination process as part of its duties.
Date modified: September 2020
A written request for re-examination must be filed with the appropriate fee (see CIPO’s website on Patent Fees) and, if the requester is a small entity, a small entity declaration (subsection 44(3) of the Patent Rules).
The request must be based on prior art consisting of patents, applications for patents open to public inspection or printed publications (subsection 48.1(1) of the Patent Act). The request must set forth the pertinence of the prior art and the manner of applying the prior art to the claim or claims for which re-examination is requested (subsection 48.1(2) of the Patent Act). For example, the request may discuss why a particular claim is rendered anticipated under section 28.2 of the Patent Act in view of a prior art document.
Any request for re-examination and the subsequent proceedings are made part of the electronic office file associated with the issued patent.
Upon receipt of a request for re-examination, a member of the Patent Appeal Board reviews the file on behalf of the Commissioner of Patents to ensure that the requirements of subsections 48.1(1) and 48.1(2) of the Patent Act and section 122 of the Patent Rules have been satisfied.
If the request satisfies these requirements and the requester is someone other than the patentee, then a package containing a copy of the request and a copy of the prior art is sent to the patentee. If the requester is the patentee, no such package is sent (subsection 48.1(3) of the Patent Act).
At the same time, the Commissioner of Patents establishes a re-examination board (subsection 48.2(1) of the Patent Act). The board must consist of not fewer than three persons, at least two of whom must be Patent Office employees. Generally, the re-examination board is composed of a Patent Appeal Board member serving as chairperson, and two patent examiners from the examination division to which the patent relates. The re-examination board members must not have participated nor advised in the examination of the application from which the patent issued.
Once the re-examination board is established, the patentee is informed of the composition of the board by the Commissioner who takes no further part in the re-examination process.[376]
Receipt of an acceptable request for re-examination and establishment of the re-examination board initiates the first stage of the re-examination process.
In the event that a request for re-examination does not satisfy the requirements of subsection 48.1(1) or 48.1(2) of the Patent Act or section 122 of the Patent Rules, the requester is so notified.
Examples of unacceptable requests are those which do not detail the pertinence of the prior art and the manner of applying said art to the claim or those which are based on material which would not qualify as “prior art” under section 48.1(1) of the Patent Act. At a minimum, an acceptable request should articulate the relationship between the features of the prior art and those of the claims for which re-examination has been requested.
A failure to include a small entity declaration if the requester is a small entity would also make the request unacceptable. However, the declaration may be submitted without resubmitting the entire request for re-examination.
Non-compliant requests for re-examination may be corrected and resubmitted without the requirement for a further fee. A request for re-examination is not considered to have been made until it is compliant with the requirements of the Patent Act and Rules. As such, no further action on the merits of the request is taken until an acceptable request is submitted.
If a request is compliant in respect to some claims but not for others, i.e. if the pertinence of the prior art is only discussed in relation to some of the claims requested for re-examination, then notification, establishment of the re-examination board and initiation of the first stage of re-examination will commence for the claims for which the request is compliant. The patentability of the claims for which the request is not compliant will not be further considered in the re-examination process.
The grant copy of a patent should never be submitted to the Patent Office when requesting re-examination.
Date modified: December 2015
Within three months of establishment, the re-examination board must determine whether a substantial new question of patentability affecting any claim of the patent for which re-examination has been requested is raised by the request for re-examination (subsection 48.2(2) of the Patent Act). This is a threshold question which must be answered before any further re-examination of the patent can continue.
In order to raise a substantial new question of patentability the request must present an issue relating to the validity of one or more claims that was not previously considered during the prosecution of the application that resulted in the patent for which re-examination has been requested, and that was not considered during any other prior proceeding involving the patent. The issue must also not be so closely related to one previously considered such that it is not a substantial new question.
A substantial new question of patentability is most often raised by prior art that was not on record during the original prosecution. However, if prior art is so similar to that which was considered during examination that it would be applied in the same manner, there would not be any material effect on the record and substantial new question of patentability would not be established.
A substantial new question of patentability may be based on the same prior art considered by an examiner during the original examination so long as the requester is able to satisfy the board that the prior art was not applied in the same manner by the examiner (i.e. that a substantial “new” question is raised). For example, a piece of prior art may have been considered during examination as having been applicable as an anticipatory reference under section 28.2 of the Patent Act. As part of a request for re-examination the same piece of prior art might be used in combination with one or more other pieces of prior art to make a case for obviousness of a claim under section 28.3 of the Patent Act.
In the absence of evidence from the record as to how an examiner considered the prior art during prosecution (e.g., the application was allowed without an office action) the board will not presume that the prior art was considered in the same manner as outlined in the request.
If the re-examination board determines that the request for re-examination does not raise a substantial new question of patentability, the requester is so notified. This notice, which takes the form of a letter from the board, will include reasons as to why the board has reached such a conclusion. Such a decision by the board is final and not subject to appeal or review by any court (subsection 48.2(3) of the Patent Act).
In the event the board determines that the request for re-examination raises a substantial new question of patentability, the patentee is so notified in a letter which includes the board’s reasons (subsection 48.2(4) of the Patent Act). These reasons are not limited to the arguments set out by the requester in the request for re-examination.
The patentee may reply within three months to the board’s notice with submissions relating to the patentability of the claim(s) of the patent for which notice was given (subsection 48.2(5) of the Patent Act). At the same time, a patentee may submit proposed amendments to the patent to address the question of the patentability of the claim(s), so that the proposed amendments are before the board for the second stage of re-examination (subsection 48.3(2) of the Patent Act).
During all stages of the re-examination proceeding, if the requester is not the patentee, the board may send the requester copies of the correspondence from the board to the patentee as a courtesy.
Date modified: October 2019
The re-examination of the patent based on the substantial new question of patentability begins upon a reply from the patentee or upon the expiration of three months from the notification from the board of a substantial new question of patentability (subsection 48.3(1) of the Patent Act).
In the event that there has been no reply from the patentee, as a courtesy the board will send a letter indicating that the re-examination of the patent has begun. The board will also advise the patentee that absent any further submissions a decision will be taken and a certificate of re-examination issued under subsection 48.4(1) of the Patent Act.
During the re-examination proceeding, there may be opportunity for multiple exchanges with the patentee in relation to the issues raised by the request. Letters from the board will set out the board’s preliminary opinions on the patentability of the claims which are subject to re-examination and any proposed amendments made by the patentee. Final determinations on patentability are reserved until the issuance of a certificate of re-examination.
The patentee may propose any amendment to the patent (subsection 48.3(2) of the Patent Act), including amendments to the description and/or drawings. Any amended or new claims proposed during re-examination must be numbered consecutively beginning with the number immediately following the number of the last claim of the issued patent (section 123 of the Patent Rules).
No amendment or new claim shall enlarge the scope of a claim of the patent (subsection 48.3(2) of the Patent Act). This provision is taken to mean that at a minimum any claim proposed during a re-examination proceeding must include all the features of the broadest independent claim of the patent. In other words, any proposed claim may not broaden the scope of protection in some respects even if the claim is narrowed in other respects.
As part of the exchanges between the board and the patentee during a re-examination proceeding the patentee may make submissions orally and/or in writing. Oral submissions may be conducted in person, via teleconference or via videoconference, at the option of the patentee.
Pursuant to subsection 48.3(3) of the Patent Act, the second stage of re-examination must be completed within twelve months.
Date modified: April 2018
The determinations of a re-examination board are functionally equivalent to a decision of the Commissioner of Patents under section 40 of the Patent Act. They are in essence a re-determination of the validity of the claims of the patent,[377] although within the particular circumstances set out in sections 48.1 to 48.5 of the Patent Act.
As such the re-examination board, like the Commissioner, must be satisfied that the patentee is not “by law” entitled to a claim of the patent in order for it to be cancelled. The same criteria would apply to a decision of the board not to incorporate a claim or other amendment into the patent with the additional requirement that such an amendment not enlarge the scope of a claim of the patent as per subsection 48.3(2) of the Patent Act.
Upon completion of the second stage of a re-examination proceeding, the re-examination board will issue a certificate of re-examination which is delivered to the patentee by registered mail (subsections 48.4(1) and 48.4(2) of the Patent Act). The certificate affects the original patent by:
If a certificate of re-examination indicates that an independent claim is cancelled from a patent, this does not mean that the text of the independent claim is no longer considered part of any dependent claim that refers to it. The cancellation of such a claim is a removal of the scope of protection afforded by the claim, not its text per se.
If any amendments have been proposed to the description and/or drawings which are determined to be permissible by the board under subsection 48.3(2) of the Patent Act, the incorporation of these amendments will be noted in the certificate of re-examination as well.
Accompanying the certificate of re-examination will be a decision from the board in the form of a letter to the patentee outlining the reasons for the board’s determinations in the certificate.
Also accompanying the certificate of re-examination will be a registration certificate indicating that the certificate of re-examination issued by the board has been registered against the patent. In this way, the certificate is attached to the patent (subsection 48.4(2) of the Patent Act).
As a result of the re-examination proceeding a new cover page is generated for the patent indicating that the patent has been re-examined. The certificate of re-examination and any amendments to the patent are stored in association with the new cover page both in the Patent Office electronic file and on the Canadian Patent Database.
Date modified: December 2015
The effects of the certificate of re-examination are set out in subsection 48.4(3) of the Patent Act, namely :
[w]here a certificate…
(a) cancels any claim but not all claims of the patent, the patent shall be deemed to have been issued, from the date of grant, in the corrected form;
(b) cancels all claims of the patent, the patent shall be deemed never to have been issued; or
(c) amends any claim of the patent or incorporates a new claim in the patent, the amended claim or new claim shall be effective, from the date of the certificate, for the unexpired term of the patent.
Thus, the invalidity of a claim or claims as a result of re-examination is retroactive. However, the addition or amendment of claims has the effect of rights only being available for those claims for the remaining term of the patent.
The above effects do not apply until the appeal period has expired (see next section), and if an appeal is taken from the board’s decision, the above effects only apply to the extent they are reflected in the final judgment of the courts (subsection 48.4(4) of the Patent Act).
Where the claims undergoing re-examination are confirmed, then said claims remain as issued for the unexpired term of the patent.
Date modified: December 2015
A decision of the re-examination board that accompanies a certificate of re-examination can be appealed by the patentee to the Federal Court (subsection 48.5(1) of the Patent Act). The appeal must be taken within three months from the date that a copy of the certificate of re-examination is sent by registered mail to the patentee (subsection 48.5(2) of the Patent Act).
Date modified: December 2015
Reissue is a mechanism provided by section 47 of the Patent Act for correcting a “defective or inoperative” patent. Subsection 47(1) of the Patent Act sets out the conditions wherein a new reissued patent may be granted to a patentee.
The purpose of the reissue provision has been described as being to provide that kind of relief which courts of equity have always given in case of clear accident and mistake in the drawing up of written instruments.[378]
It is important to note that in accordance with the provision that the Commissioner of Patents “may” cause a new patent to be granted, the granting of a reissue is a discretionary measure. However, such discretion can only be exercised once the conditions of subsection 47(1) have been met. Any application for reissue which does not fall within the statute must be refused.[379] Any such exercise of discretion must also be compatible with the purpose of the reissue provision, as noted above.[380]
Date modified: October 2019
Subsection 47(1) of the Patent Act requires that a patent be surrendered within four years from its date of grant in order to obtain a reissue. This has been interpreted as requiring that an application for reissue be filed within four years from the grant of the original patent.[381] The surrender of the original patent referred to in subsection (1) is considered to take place at the time the application for reissue is submitted in accordance with Form 1 of Schedule 1 of the Patent Rules, but only to take effect if a new patent is issued.
The original patent should never be returned to the Office for the purpose of a reissue.
Date modified: April 2018
Pursuant to subsection 47(1) of the Patent Act, the Commissioner may cause the issue of a new or amended patent (a “reissue patent”) whenever a patent is deemed “defective or inoperative”. At a minimum, this means that, due to some error, the original patent failed to fulfil the applicant’s intent upon grant.[382] As a result, the patentee has been granted a patent that fails to represent that which the applicant truly intended to have been covered and secured by it. The words “defective or inoperative” in the sense used in subsection 47(1) of the Patent Act do not equate to a “defect” in relation to the compliance of a patent with the Patent Act and Rules for validity purposes. A patent may be valid in all other respects, yet nonetheless fail to express what the applicant had intended.
Under subsection 47(1) of the Patent Act, there are two reasons why a patent can be deemed defective or inoperative: (1) insufficient description and specification; and (2) the patentee having claimed more or less than the patentee had a right to claim as new. Thus, the “defectiveness or inoperability” of the patent potentially affects the scope of protection of the patent or how well the patent describes the invention.
The words “defective or inoperative”, however, do not encompass the reissue of a patent that has been judicially declared invalid,[383] or a patent for which all rights have lapsed.
An application for reissue is not a means for reopening the prosecution and permitting a patentee to amend a patent as they would amend a patent application during its normal prosecution.[384] Nor does it permit a patentee to unilaterally narrow the scope of protection as would filing a disclaimer—an application for reissue must satisfy the requirements of subsection 47(1) of the Patent Act.
Date modified: October 2019
The reissue provision exists to correct an “error” that “arose from inadvertence, accident or mistake, without any fraudulent or deceptive intention”
(subsection 47(1) of the Patent Act). Such an error must be one whereby the patent for which reissue is sought fails to express the intended invention;[385] that is the patent fails to state something or misstates something.
A patentee seeking reissue must show that due to “inadvertence, accident or mistake” a result that was other than what was intended by the applicant – as of the date of issuance – occurred. Mere support in the original patent for the proposed amendments is insufficient to establish intent.[386] The patentee must establish that the issued patent does not accurately express the applicant’s intention with respect to the description and specification of the invention. Similarly, in cases where an original patentee has assigned his right to a patent, the assignee must still establish that the issued patent does not accurately express the intent of the applicant of the original patent. In all cases, if it is obvious that the intent of the applicant was completely fulfilled, a reissue is not justified.[387]
This is usually the most difficult part of the reissue provisions to satisfy, as there must be evidence that an error did in fact occur during the prosecution of the original patent. There also must be evidence of what the applicant had intended the original patent to say.
Mere allegation of an error is not evidence of that error. It is not evidence from which the Commissioner or a court can conclude that an error was made.[388]
It is the evidence as a whole that is considered; such evidence can include, for example, the text of the patent itself, evidence of actions taken during the prosecution of the original patent application[389] and of corresponding patent applications in this and other jurisdictions[390] and evidence of communications indicating intended actions that were never taken, etc. To be of practical use, the evidence must pre-date the issuance of the original patent.[391]
The onus lies with a patentee to prove that an error occurred during the prosecution of the patent application through inadvertence, accident or mistake; the extent of prior patent experience of the applicant/appointed patent agent may be taken into consideration.[392] It is presumed that the applicant’s intent has been fulfilled by the issued patent,[393] which presumption may be rebutted by sufficient evidence to the contrary. For example, a patentee may allege that certain claims were cancelled from an application by mistake, but the record may show that this was done in the face of an identified defect from an examiner or to avoid conflict or to avoid prior art.[394] Unless a patentee can prove that such an action was not to have been taken, it is presumed to be indicative of the applicant’s intent.
The intent has to be that of the applicant, but the error could have been made or caused by anyone (which can include the patent agent or applicant) during prosecution of the original patent.[395]
A mistake in interpreting the law may lead to an error in a patent. However, in order to fall within subsection 47(1) of the Patent Act such an error must have led to a patent which fails to represent the applicant’s intent.[396]
Date modified: December 2015
A patent defective or inoperative for insufficient description and specification is one lacking textual or graphical matter or including the wrong textual or graphical matter, contrary to the intent of the inventor upon grant. A failure to: accurately claim or describe the invention; claim subcombinations; include dependent claims; or, include claims to different categories of invention could be indicative of a patent defective or inoperative for insufficient description and specification.
Date modified: September 2020
A patent defective or inoperative by reason of the patentee claiming more or less than he had a right to claim as new is one that results in the claims protecting more or less subject-matter than the patentee had intended. Since the description and drawings affect the scope of the claims, an error involving these parts of the patent could also result in the patent being defective or inoperative for claiming more or less than the patentee had a right to claim.[397]
Date modified: December 2015
Whatever defect a patentee seeks to rectify in the patent by an application for reissue, any “amended description and specification” made by the patentee must be directed to the “same invention ... for which the original patent was granted” (subsection 47(1) of the Patent Act).
Although section 38.2 of the Patent Act does not apply to the reissue process, its requirements (see chapter 20 of this manual) are considered to be analogous to those of subsection 47(1) for a reissued patent to be for the “same invention”.
Accordingly, all matter in a reissued patent must find support somewhere in the description, drawings or claims of the original patent.[398] However, there is no requirement that an invention sought to be protected by reissue need be directed to the same inventive concept as the claims of the original patent.[399]
Subject-matter sought to be added that is inferable from the original description, drawings or claims would comply with the “same invention” requirement of subsection 47(1) of the Patent Act.[400] Matter that is admitted to be prior art would be acceptable as well.[401]
Date modified: October 2019
An application for reissue must include a Form 1 of Schedule 1 of the Patent Rules, completed as per the instructions (section 118 of the Patent Rules). Sections 3, 4 and 5 of Form 1 set out the patentee’s case for granting a reissued patent. The application for reissue must also include an amended description, set of drawings and/or set of claims. All changes introduced by the amendments must be consistent with the defects identified in Form 1.[402] The patentee should also submit the most relevant available evidence (see section 31.01.02a of this manual).
The original patent should never be returned to the Office for the purpose of a reissue.
Date modified: October 2019
In section 3 of Form 1 the patentee must identify specifically how the patent is defective or inoperative. This discussion can refer to problems with the claims, description or drawings. The issues must be linked with an insufficiency of description and specification or the patentee having claimed more or less than he had a right to claim as new (see sections 31.01.03 – 31.01.04 of this manual).
Section 4 of Form 1 must illustrate how the error arose which led to the patent specifying something other than what was intended. It is here that the patentee must demonstrate that an error occurred, in the sense that the patent document does not accord with the intent of the applicant[403] (see section 31.01.02a of this manual). Section 4 of Form 1 should refer to any applicable submitted evidence when explaining how the error arose and how the patent document does not accord with the intent of the applicant.
Section 5 of Form 1 details when and how the patentee became aware of the error leading to the application for reissue. The error must have been discovered after the patent was issued[404] since if an applicant allowed a patent to grant with full knowledge that an error had occurred during the prosecution, such an act would normally be taken as a deliberate one and thus as reflecting the applicant’s intent. In some cases wherein the discovery of the error occurred after payment of the final fee but before issue of the patent an application for reissue may also be acceptable given an appropriate explanation. Note that the circumstances described in section 5 of Form 1 may also be relevant to the determination of whether the applicant’s intent was truly unfulfilled by the original patent.
Date modified: October 2019
Unlike disclaimers, an application for reissue can result in the widening of a patent’s scope; the scope of protection can be increased, thereby affecting the bargain with the public. The Patent Office must therefore examine and approve any application for reissue.[405]
Given the retroactive effect of reissued patents (subsection 47(2) of the Patent Act), it is important to ensure that an application for reissue meets the requirements of section 47 of the Patent Act.[406] The Reissue Board, consisting of senior patent examiners from the various examination disciplines, oversees the complete application for reissue process and is tasked with ensuring that applications for reissue meet those requirements.
For any submitted application for reissue the Reissue Board first verifies that the application for reissue complies with the requirements of section 47 of the Patent Act as detailed in sections 31.01.02 - 31.01.05 of this manual. This verification will involve a review of the application, proposed amendments and submitted evidence as well as the record of prosecution in the Patent Office and, if applicable, foreign patent offices. If the application complies with the requirements then an examiner of the relevant art verifies that the reissued patent would comply with the rest of the Patent Act and Rules. This verification is analogous to the examination of a patent application and may identify any defect that would be applicable during said examination.
If the Reissue Board or examiner finds the application for reissue to be noncompliant with the Patent Act and Rules, an office letter explaining why will be issued. The patentee may respond by arguing and clarifying points, providing further evidence (which will be put on file), and/or by amending the proposed description, drawings and/or claims. The response should address the issues identified in the office letter.
Form 1 may not be amended . Section 47 of the Patent Act does not permit amendments to Form 1 that change the reasons for reissue.
If an impasse is reached between the patentee and the Reissue Board or the examiner, the application for reissue may be referred to the Patent Appeal Board, who will make a recommendation to the Commissioner of Patents.
A patentee should present the best evidence upon filing Form 1. All issues and evidence should be before the Reissue Board and examiner prior to any referral to the Patent Appeal Board. Further, before an application is forwarded to the Patent Appeal Board, all deficiencies therein with regards to the Patent Act and Rules must have been identified by the Reissue Board and examiner, even if an impasse occurs in the initial stage of examination before the Reissue Board.
An application for reissue does not go abandoned for failing to respond to an office letter within a certain time period, and thus there are no corresponding reinstatement fees. On the other hand, failure to respond in a timely manner can result in the application for reissue being forwarded to the Patent Appeal Board and the Commissioner of Patents.
A patentee may always end prosecution by withdrawing the application for reissue.
If the application for reissue is found acceptable by both the Reissue Board and the examiner, the patent will be reissued with an “E” document code. If the original patent was issued on the basis of an application filed after October 1, 1989 then the reissued patent will have the same patent number as the original patent; original patents issued on the basis of applications filed before said date will be reissued with a new patent number in the one million series. If the application for reissue is found acceptable the Office will send the patentee the new patent.
Date modified: April 2018
A patentee may file separate applications for reissue in respect of distinct parts of the invention covered by the original patent being reissued (subsection 47(3) of the Patent Act). This could result in multiple reissued patents. As with other applications for reissue, each separate application must be filed within four years of the original grant date. The separate application for reissue must all have been filed before the effective date of surrender of the original granted patent, i.e. before the grant of a reissued patent based on any one of them.
This subsection is permissive in that it allows the patentee to file multiple applications for reissue. The Commissioner of Patents will not call for division of an application for reissue, whether the granted patent appears to have been granted with more than one invention, or an additional invention is being claimed by reissue so that the reissue will contain more than one invention. Under 36(2.1) of the Patent Act, the Commissioner can only call for division of a patent application before the issue of a patent on the original application. Similarly, subsections 36(2), 36(3) and 36(4) of the Patent Act also do not apply to applications for reissue.
Each separate application for reissue under subsection 47(3) of the Patent Act must be independently patentable as covering separate inventions in order to ensure that double patenting does not arise. Where multiple applications for reissue co-exist for the same patent, yet do not cover separate and distinct parts of its invention, only one reissued patent (at most) can be granted.
While separate applications for reissue may be filed under subsection 47(3) of the Patent Act, the requirements of subsection 47(1) of the Patent Act must still be met in order to justify reissue. That is, an error must have occurred which led to the intent of the applicant of the original patent not having been fulfilled, which now results in the necessity of two or more separate reissued patents.
Date modified: April 2018
A situation could arise in which one or more co-existing applications for reissue are considered compliant with the Patent Act and Patent Rules, but at least one co-existing application for reissue for the same patent is refused by the Commissioner of Patents or withdrawn by the patentee. If applicable in such a case, the patentee will be notified that they may, if desired and before the expiry of a specified time limit, introduce subject-matter that appears in the refused or withdrawn application for reissue into one of the compliant applications for reissue as long as that subject-matter appeared in the original patent. If, after the introduction of subject-matter, the previously-compliant co-existing application for reissue remains compliant with the Patent Act and Patent Rules, then a patent will proceed to be reissued for the compliant applications for reissue. If the introduction of subject-matter causes the previously-compliant, co-existing application for reissue to fail to comply with the Patent Act and/or Patent Rules, then examination will continue on the previously-compliant, co-existing application for reissue until it is found to be compliant, is refused or is withdrawn. If no response is received within the specified time limit, then patents will be reissued based on the compliant applications for reissue as they exist on file in the Office.
Date modified: December 2015
A reissued patent may itself be reissued provided that the application to reissue is filed within four years of the date of grant of the original patent (not of the reissued patent), and provided that the invention sought to be protected by reissue is directed to the same invention for which the original patent was granted.
Date modified: October 2019
The effect of reissue is retroactive and rights exist with respect to the reissued patent as if they had been in effect as of the original grant date (subsection 47(2) of the Patent Act).
A reissued patent may not be withdrawn after it has been issued in favour of the original patent.
Any pending action is not affected by a reissue “to the extent that its claims are identical with the original patent”
(subsection 47(2) of the Patent Act). In this context “identical” is taken to mean “of the same scope”.[407]
No additional maintenance fees apply to an application for reissue (sections 112 and 113 of the Patent Rules). However, maintenance fees remain payable on the original patent until it reissues (if it reissues), and then become payable on the reissued patent under the same conditions as the original patent (section 114 of the Patent Rules), that is, in accordance with the maintenance fee due dates that apply to the original patent (see chapter 8 of this manual).
Date modified: December 2015
Although not explicitly provided by the Patent Act, a refusal to grant a reissue by the Commissioner of Patents is subject to appeal to the Federal Court under section 41 of the Patent Act.[408]
Date modified: December 2025
The term of a patent is the length of time for which a patentee is entitled to exclusive rights and privileges to the invention. The standard term of a patent is 20 years from its filing date. This term can expire earlier if, for example, the patentee does not pay the annual maintenance fees.
An additional term may be granted by the Commissioner of Patents. An additional term compensates patentees for unreasonable delays in the processing of their patent applications. Additional term begins on the expiry of the 20-year standard term of an eligible patent, but only if the patent remains valid until expiry of this standard term.
An additional term is distinct from a certificate of supplementary protection (CSP) issued by the Minister of Health. CSPs are only for patents claiming new medicinal ingredients or new combinations of medicinal ingredients. Should a patent be granted both an additional term and a CSP, these terms would both begin upon the expiry of the standard term of the patent and would run concurrently.
Date modified: December 2025
Under paragraphs 46.1(1)(a) to (c) of the Patent Act, to be eligible for additional term, criteria relating to key dates (such as date of issuance of the patent, the filing date of the application for the patent, and additional term application date) must be met. In addition, the patentee must meet additional term application requirements must be met.
Date modified: December 2025
An additional term is only applicable for patents if the application for the patent was filed on or after December 1, 2020 (paragraph 46.1(1)(b) of the Patent Act).
In addition, the patent must have been issued after the date that is determined to be the later of the following dates (the “Later Date”):
Date modified: December 2025
Example 1: PCT national entry application with a filing date on or after December 1, 2020.
The Later Date is the later of five years from the national entry date, and three years from the date of the request for examination.
The national entry date is May 30, 2023. The request for examination date is November 29, 2024.
The later of:
The Later Date of the two dates is May 30, 2028.
Example 2: Divisional application with a filing date on or after December 1, 2020.
The Later Date is the later of 5 years from the presentation date, and 3 years from the date of the request for examination.
The presentation date is June 1, 2023. The request for examination date is June 1, 2023.
The later of:
The Later Date of the two dates is June 1, 2028.
Example 3: Any application other than a PCT national entry application or a divisional application, with a filing date on or after December 1, 2020
The Later Date is the later of 5 years from the filing date, and 3 years from the date of the request for examination.
The filing date is December 1, 2020. The request for examination date is December 1, 2023.
The later of:
The Later Date of the two dates is December 1, 2026.
Date modified: December 2025
If applying for additional term, the patentee must submit a compliant application for additional term and pay the fee within three months after the issue date of the patent. The application must be made in writing, and the applicable patent number must be indicated (paragraph 46.1(1)(c) of the Patent Act, subsection 117.01(1), (3) of the Patent Rules).
An error in the patent number provided in the application may be corrected before the later of
Under subsection 117.01(4) of the Patent Rules, only one application may be submitted for a given patent. However, if an application for additional term is dismissed for failing to meet the eligibility requirements under paragraphs 46.1(1)(a) to (c) of the Patent Act, the application for additional term is deemed never to have been made. If this happens, the patentee may apply again.
Date modified: December 2025
If the eligibility criteria under paragraphs 46.1(1)(a) to (c) of the Patent Act are not met, the application for additional term is dismissed and is deemed never to have been made. The Commissioner will send a notice to that effect (subsection 117.01(5) of the Patent Rules).
Date modified: December 2025
If an application for additional term has not been dismissed for failing to meet the eligibility requirements under paragraphs 46.1(1)(a) to (c) of the Patent Act, the process for determination of additional term will continue.
Date modified: December 2025
The Commissioner will send a notice to the patentee indicating the Commissioner's preliminary determination of the duration of the additional term, if any (subsection 117.01(6) of the Patent Rules).
Date modified: December 2025
The patentee has two months from the date of the notice containing the preliminary determination to provide observations regarding the preliminary determination (subsection 117.01(7) of the Patent Rules). Observations will be considered by the Commissioner when making a determination of additional term.
Date modified: December 2025
After the period for making observations, the Commissioner will issue either:
The Commissioner will provide reasons for the duration of the additional term, or for the dismissal of the application, as the case may be (subsection 117.01(8) of the Patent Rules).
Date modified: December 2025
A certificate of additional term sets out the number of days of additional term and must include
The Commissioner is authorized to correct any typographical error in the certificate. The certificate will be available on the Canadian Intellectual Property Office website (subsections 117.04(2) and (3) of the Patent Rules).
Date modified: December 2025
The duration of additional term contained in the preliminary determination is the result of applying a calculation that takes into account the number of days between the Later Date (see section 32.02.01) and the issue date of the patent less the number of days to be subtracted that are determined under the Patent Rules (subsection 46.1(4) of the Patent Act, section 117.03 of the Patent Rules).
When calculating the number of days between the Later Date and the issue date of the patent, the Later Date is excluded, and the day of issuance is included.
Example:
Later Date: January 20, 2026
Issue date of the patent: July 14, 2026
Number of days between the Later Date and the issue date = 175 days
In the example above, 175 days is the number of days used in the calculation of the duration of additional term before days are subtracted under the provisions of the Patent Rules.
Date modified: December 2025
The days to be subtracted are the total number of days within all applicable periods of time determined under the section 117.03 of the Patent Rules.
Date modified: December 2025
Subsection 117.03(1) of the Patent Rules defines periods reflecting events during the history of a patent application. There may be multiple applicable periods having days to be subtracted, and a particular period may apply more than once. Days in one period may overlap with those in another. However, in the determination of additional term, a calendar day may be subtracted only once. The number of days in any given period of subtracted days includes the first day but does not include the last day (subsections 117.03(2), (5) and (6) of the Patent Rules).
If the day on which a period ends is on or before the day on which the period begins, the number of days in the period is zero. If the period does not end before the patent is issued, then the period ends on the date of issuance of the patent (subsections 117.03(10), (11) of the Patent Rules).
The period of time beginning on the applicable day referred to in subsection 46.1(2) of the Patent Act and ending on the earlier of the date of a compliant request for examination and the day the application is deemed abandoned for failing to request examination will be subtracted (paragraph 117.03(1)(k) of the Patent Rules). However, if the Later Date is determined to be the third anniversary of the request for examination date (rather than the fifth anniversary of the applicable day referred to in subsections 46.1(1) and (2) of the Patent Act), then subtracted days do not include any period of time prior to the request for examination date (subsection 117.03(8) of the Patent Rules).
Any period of time during which an application is deemed abandoned will be subtracted (paragraph 117.03(1)(x) of the Patent Rules).
If an extension of time is granted under subsection 3(1) of the Patent Rules, the period begins on the original deadline and ends on the earlier of the day the action for which the extension was requested is taken, and the last day of the extension (paragraph 117.03(1)(j) of the Patent Rules).
Date modified: December 2025
If a notice is sent to an applicant or other person requiring them to take an action in respect of the patent application, the days in an applicable period of time will be subtracted. In the majority of cases, the period of time begins on the date of the notice. Depending on the type of notice, the period will end on specified day (such as a deadline day), or on the earlier of two or more days (such as the earlier of the day a specified action is taken and the day the application is deemed abandoned). The Patent Rules also identify the number of days to subtract in the case where a notice is withdrawn, and where a notice was erroneously not sent by the Patent Office.
Date modified: December 2025
If a notice of non-compliance is sent to an applicant under section 65 of the Patent Rules, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice requiring the applicant to pay the application fee under is sent under subsection 27(7) of the Patent Act, the period begins on the date of the notice and ends on the day of payment of the application fee and the late fee, or, if they are paid on different days, the later of those days (paragraph 117.03(1)(d) of the Patent Rules).
Date modified: December 2025
If a notice requiring translation of the specification is sent to an applicant under subsection 15(4) of the Patent Rules, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice requiring a copy of the priority application is sent to an applicant under subsection 74(4) of the Patent Rules, the period begins on the date of the notice and ends on the earliest of
Date modified: December 2025
If a notice requiring a translation of the priority application is sent to an applicant under subsection 76(1) or (2) of the Patent Rules, the period begins on the date of the notice and ends on the earliest of
Date modified: December 2025
When an examiner’s requisition or final action is sent to an applicant under subsections 85(1), (2), subsections 86(2), (5), or section 94 of the Patent Rules, the period begins on the date of the requisition or notice and ending on the earlier of:
Date modified: December 2025
If a notice requiring the applicant to request continued examination was sent under subsection 85.1(1) of the Patent Rules, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice of allowance sent to the applicant under subsection 86(1), (6), (10), or (12) of the Patent Rules, the period begins on the date of the notice and ends on the earliest of
Date modified: December 2025
If a conditional notice of allowance sent to the applicant under subsection 86(1.1) of the Patent Rules, the period begins on the date of the notice and ends on the earliest of
Date modified: December 2025
If a notice was sent to the applicant indicating that submissions or proposed amendments may be made on or before a specified day is sent on or after the day on which the Commissioner sends a notice indicating that the rejection is not withdrawn under paragraph 86(7)(a) of the Patent Rules, the period begins on the date of the notice and ends on the earliest of
Date modified: December 2025
If a notice proposing a hearing date is sent to the applicant on or after the day on which the Commissioner sends a notice indicating that a rejection is not withdrawn under paragraph under paragraph 86(7)(a) of the Patent Rules and the hearing is rescheduled for a later date at the request of the applicant, the period begins on the date of the proposed hearing and ends on the earlier of
Date modified: December 2025
If, after review of a rejected application, a notice is sent under subsection 86(11) of the Patent Rules to inform the applicant that amendments must be made and the applicant makes amendments to the application after the date of the notice, other than amendments made in response a final action, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice requiring the appointment of a patent agent is sent to the applicant, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice of disregarded communication is sent under subsection 40(1) of the Patent Rules to a joint applicant who is not the common representative, the period begins on the date of receipt of the written communication from that joint applicant and ends on the earliest of
Date modified: December 2025
If a notice of disregarded communication is sent under subsection 41(1) of the Patent Rules to a patent agent who is not appointed to represent the applicant, the period begins on the date of receipt of the written communication from that patent agent and ends on the earliest of
Date modified: December 2025
If a notice of disregarded communication is sent under subsection 41.1(2) of the Patent Rules to the sender of a communication in which a patent agent is not named, the period begins on the date of receipt of the written communication from sender and ends on the earliest of
Date modified: December 2025
If a notice is sent under subsection 154(7) of the Patent Rules to a person requiring them to establish that they are either the applicant of the international application or their legal representative, the period begins on the date of the notice and ends on the day the person complies with the notice (paragraph 117.03(1)(z.03) of the Patent Rules).
Date modified: December 2025
If a notice is sent to the applicant under subsection 155.5(6) of the Patent Rules requiring translation of parts of a PCT national phase application or a complete copy of translated parts of the PCT national phase application, the period begins on the date of the notice and ends on the earlier of
Date modified: December 2025
If a notice is sent to the applicant under any provision of the Patent Act or Rules requiring them to take an action within a specified period, other than a notice referred to in any of paragraphs 117.03(1)(a), (c) to (e), (h), (i), (l), (m), (q) to (u) and (y) to (z.04) of the Patent Rules, the period of time begins on the date of the notice and ends on the earliest of
Date modified: December 2025
The Patent Rules require the subtracting of periods of time following a request from an applicant to withdraw a notice in certain circumstances (paragraphs 117.03(1)(z.05)-(z.07) of the Patent Rules). If a communication sent by the Commissioner or the Patent Office is withdrawn, the communication is considered never to have been sent for the purposes of determining periods to subtract, except for the purposes of determining the periods calculated under paragraphs 117.03(1)(z.05)-(z.07) (subsection 117.03(9) of the Patent Rules).
Date modified: December 2025
In the case where a notice to the applicant appears in the records associated with the application for the patent on the website of the Canadian Intellectual Property Office yet the notice was not sent to the applicant because of an error made by the Patent Office, a period of time will be subtracted in accordance with paragraph 117.03(1)(z.08) of the Patent Rules. However subtraction of this period will not apply if the applicant notifies the Commissioner in writing that they did not receive the notice and on the same day files a statement that they are notifying the Commissioner without undue delay after they became aware of the notice and the fact that they had not received it (subsection 117.03(12) of the Patent Rules; see also sections 12.04.03 and 2.02.09 of this manual).
Date modified: December 2025
Certain events during the application phase or in the course of prosecution, unrelated to notices described in section 32.04.03 of this manual, will result in periods of time to be subtracted.
Date modified: December 2025
If the Commissioner requires further drawings under subsection 27(5.2) of the Patent Act, the period begins on the date the request is made and ends on the earlier of
Date modified: December 2025
If an application maintenance fee was not paid under subsection 27.1(1) of the Patent Act on or before the prescribed date to pay the fee, the period begins on the prescribed day to pay the fee and ends on the earlier of
Date modified: December 2025
If an applicant submits an addition and statement under subsection 28.01(1) of the Patent Act, the period begins on the filing date of the application and ends on the later of
Date modified: December 2025
If an applicant requested further examination prior to October 3, 2022 or requested continued examination, the period begins on the day of the request for further examination or the request for continued examination and ends on the day of payment of the final fee, or if the final fee has been refunded, the day on which the final fee was paid again (paragraph 117.03(1)(n) of the Patent Rules).
Date modified: December 2025
If, on or before October 2, 2022, a compliant request for examination was made and a total of three examiner notices were sent under subsection 86(2) (notice of defects) or subsection 86(5) (final action) of the Patent Rules before a notice of allowance or conditional notice of allowance was sent, the period begins on the date of the third such notice and ends on the day of payment of the final fee, or if the final fee has been refunded, the day on which the final fee was paid again (paragraph 117.03(1)(o) of the Patent Rules).
Date modified: December 2025
If, in an interview initiated by an examiner, the applicant agreed to consider making an amendment to correct defects identified by the examiner on or before a specified day, the period begins on the date of the interview and ends on the earliest of
Date modified: December 2025
If the Commissioner directed the applicant to limit the claims of the application to one invention only, the period begins on the date of direction and ends the earlier of
Date modified: December 2025
If an applicant appeals a refusal under section 41 of the Patent Act or makes an application for judicial review of a decision of the Commissioner or the Patent Office in respect of a patent application, the period begins on the date of the notice of refusal or date of issuance of the notice of application for judicial review, respectively, and ends on the date of the outcome in the relevant Court described in paragraphs 117.03(1)(w), (z.1) of the Patent Rules (e.g. final judgment, date of discontinuance, etc.).
If a court orders a stay of the examination or prosecution of the application, the period begins on the day the order is made and ends on the day that the stay is no longer in force (paragraph 117.03(1)(z.11) of the Patent Rules).
Date modified: December 2025
The Commissioner may designate a day during which the operations at the Patent Office are significantly disrupted for all or part of the day during ordinary business hours due to circumstances beyond the control of the Patent Office. All designated days are subtracted in the calculation of the duration of additional term (paragraph 117.03(1)(z.12), subsection 117.03(3) of the Patent Rules).
A list of designated days is published on the Canadian Intellectual Property Office website (subsection 117.03(4) of the Patent Rules).
Date modified: December 2025
The Commissioner may reconsider the duration of an additional term granted under the Patent Act. The duration of an additional term can only be shortened through the reconsideration (i.e. it cannot be lengthened through the reconsideration process). The duration of an additional term may be reconsidered on the Commissioner’s own initiative or on application by any person (subsection 46.3(1), (4) of the Patent Act).
Date modified: December 2025
A reconsideration may be initiated by the Commissioner. This may be done in the case where it is suspected that an error in the calculation of the duration of the additional term resulted in the granting of an additional term that is longer than is authorized under the Patent Act. No fee applies in the case of a reconsideration that is initiated by the Commissioner.
Date modified: December 2025
An application for reconsideration can be made any time on or after the date that an additional term is granted. An application for reconsideration of the duration of an additional term must be in writing. The application must contain:
An application for reconsideration must be accompanied by the prescribed fee (subsection 46.3(2) of the Patent Act, subsection 117.11(2) of the Patent Rules).
If the application requirements are not met, the Patent Office will send a letter informing the person who applied for reconsideration of the missing requirements. The applicant for reconsideration may submit the missing requirements after the letter is sent. If the application requirements are ultimately met, a preliminary determination of reconsideration will follow.
The Commissioner may stay a reconsideration pending the conclusion of any court proceeding (subsection 46.3(6) of the Patent Act).
Date modified: December 2025
The patentee will receive a notice of preliminary determination after a compliant application for reconsideration is received, or after the Commissioner has initiated a reconsideration of additional term. If the reconsideration is initiated by a person other than the patentee, that person will also receive the notice of preliminary determination.
The notice will indicate
Date modified: December 2025
The patentee and the person applying for reconsideration, if applicable, can make observations within two months of the notice of preliminary determination (subsection 117.11(7) of the Patent Rules). Observations will be considered by the Commissioner.
Date modified: December 2025
After the period for making observations, the Commissioner will either
The Commissioner will provide to the patentee and, if applicable the person who applied for reconsideration, reasons for the shortened duration of the additional term indicated in the amended certificate, or for the dismissal of the application for reconsideration, as the case may be (subsection 117.11(8) of the Patent Rules).
Date modified: December 2025
A person may bring an action in the Federal Court against a patentee to shorten the duration of an addition term (subsection 46.4(1) of the Patent Act).
If the Court finds that the duration of the additional term granted is longer than is authorised under the Patent Act, the Court will, by order, shorten the duration (subsection 46.4(2) of the Patent Act).
Date modified: December 2025
If the Court shortens the duration of the additional term, a certified copy of the Court’s order will be sent to the Commissioner. The Commission will issue an amended certificate of additional term based on the Court’s order and send it to the patentee (subsection 46.4(3) of the Patent Act).
Date modified: October 2022
Transitional provisions in the amended Patent Act and the Patent Rules (SOR/2019-251) aim to preserve rights and deadlines that applicants and patentees benefited from under previous patent legislative regimes.
A series of transitional provisions in the Patent Act (sections 78.1 to 78.6) and the Patent Rules (sections 165 to 235) define the conditions and requirements for filing, prosecution and maintenance of existing patent applications and patents following the transition to the new patent legislative regime on October 30, 2019.
The transitional provisions in the Patent Rules define two categories of patent applications that are subject to transitional provisions. They are:
Determining which set of provisions of the Patent Act and/or the Patent Rules applies may be based on:
Deadlines to respond to notices sent before October 30, 2019, the coming-into-force date (CIF) of the Patent Rules (SOR/2019-251), will remain unchanged after October 30, 2019.
This chapter focuses mainly on transitional provisions and other rules concerning Category 3 applications, and current valid patents with filing dates before October 30, 2019.
The chapter also includes transitional provisions due to the coming-into-force of the College of Patent Agents and Trademark Agents Act and College of Patent Agents and Trademark Agents Regulations (SOR/2021-131) on June 28, 2021 (see section 33.05.04) and transitional provisions due to the coming-into-force of certain provisions of the Patent Rules amending the Patent Rules (SOR/2022-120) on October 3, 2022 (see section 33.13).
Date modified: October 2022
The dates accorded to communications sent or received is governed by the Patent Rules in effect on that date. Note that as of October 30, 2019 and in accordance with section 8.1 of the Patent Act and subsection 10(4) of the Patent Rules, it will be possible to establish a date of receipt for communications submitted to the Office on days it is closed if they are submitted electronically. For more information, please see Chapter 2.
Date modified: October 2022
Under the rules as they read prior to October 30, 2019 (“former Patent Rules”), the Commissioner must have regard to documents in a language other than English or French if they are submitted or made available with respect to Category 3 applications in circumstances outlined in section 215 of the Patent Rules:
Date modified: October 2022
An extension of time authorized by the Commissioner under sections 26, 26.1 and 27 of the former Patent Rules to extend time for doing something that ends after October 30, 2019 remains valid after October 30, 2019.
Under transitional provisions in sections 212 and 213 of the Patent Rules (SOR/2019-251), the Commissioner may authorize extensions of time for the following requisitions sent before October 30, 2019 where the time to respond in good faith ends after that date:
The request for extension of time must be submitted before the expiry of the original time limit and the requestor must pay the prescribed fee, which can be found on CIPO’s webpage for Patent Fees. In order for the Commissioner to be satisfied that the circumstances justify the extension, the applicant must provide a simple justification that explains why an extension of time is being requested. No evidence or affidavit is required when requesting an extension of time. The Office will assess the request and if it is compliant and reasonable, the Commissioner will generally grant an extension of time of up to six months per file and per action. The applicant/patentee will be notified by letter of the Commissioner’s decision regarding any request for an extension of time for time periods which can be extended. For information on the service standard for this request, please refer to CIPO’s website.
Date modified: October 2019
With respect to Category 3 applications, the Commissioner may grant an extension of time to ‘top up’ specified fees previously paid at the small entity rate under sections 189, 190, subsection 207(2) and section 211 of the Patent Rules (SOR/2019-251). For information about the requirements that must be met in order for an extension of time to be granted, please see chapter 2.03.03 of this manual. Note that the Commissioner will not grant an extension of time to ‘top up’ fees paid at the small entity rate prior to June 2, 2007.
Date modified: October 2019
The Patent Act contains transitional provisions related to the filing date of patent applications as summarized below.
Date modified: October 2019
Applications filed before October 30, 2019 that do not receive a filing date before the October 30, 2019 will be deemed never to have been filed as outlined section 78.21 of the Patent Act.
Date modified: October 2019
Divisional applications that were submitted before October 30, 2019 are not subject to the divisional requirements in section 89 of the Patent Rules (SOR/2019-251).
If the documents and information required to establish a presentation date under subsection 103(1) the Patent Rules (SOR/2019-251) was received by the Commissioner and at least one of those elements was received after the October 30, 2019, the presentation date is the date that the last requirement was received (sections 188, 202, 231 of the Patent Rules (SOR/2019-251)).
Date modified: October 2019
The Patent Rules contain transitional provisions related to compliance requirements for applications as summarized below.
Date modified: October 2019
With respect to the page numbering of the specification (description and claims), Category 3 applications may comply with the provisions of the former Patent Rules rather than those of section 193 of the Patent Rules. This means that the pages of the description may be numbered separately from the pages of the claims, as outlined in the former Patent Rules.
Category 3 applications with a filing date prior to June 2, 2007, may comply with the requirements for sequence listings found in the Patent Rules as they read immediately prior to June 2, 2007. Amendments to the Patent Rules made on June 2, 2007, brought the formatting requirements for sequence listings in the Patent Rules in line with the Patent Cooperation Treaty (PCT) standard.
Date modified: October 2019
For applications filed before October 30, 2019, an applicant may, instead of complying with the requirements to submit inventor and entitlement information in section 54 of the Patent Rules (SOR/2019-251), comply with the requirements surrounding submission of inventor and entitlement information that are in the former Patent Rules. Applicants choosing to comply with the former Patent Rules must consult the relevant ‘point-in-time’ version of the former Patent Rules, as described in Patent Rules (SOR/2019-251).
In addition, applicants of PCT national phase applications may file a declaration as to the applicant’s entitlement on the filing date to apply for and be granted a patent in accordance with Rule 4.17 of the Regulations under the PCT (sections 225, 226, 227 of the Patent Rules (SOR/2019-251)).
Date modified: October 2019
If, after October 30, 2019, an applicant fails to respond in good faith to a requisition sent before October 30, 2019 under sections 23, 25, 37 or 94 of the former Patent Rules, section 73 of the former Patent Act, as it read before October 30, 2019, will apply to the abandonment resulting from the failure (subsection 78.52(2) of the Patent Act).
Date modified: October 2019
The Patent Rules contain transitional provisions related to representation of applicants and patentees as summarized below.
Date modified: September 2020
Regarding patent applications, if there are joint applicants and no patent agent is appointed immediately prior to October 30, 2019, a common representative will be appointed by default, under the transitional provisions of the Patent Rules, as follows:
If no patent agent was appointed in respect of the application at any time prior to October 30, 2019, the authorized correspondent under the former Patent Rules is deemed to be the common representative under paragraph 218(a) of the Patent Rules. If there was a patent agent appointed in respect of the application, but the appointment was revoked prior to October 30, 2019, the joint applicant whose name appears first in alphabetical order is deemed to be the common representative under paragraph 218(b) of the Patent Rules.
Furthermore, if there was a patent agent appointed in respect of a patent application as of October 30, 2019, but this appointment is subsequently revoked, the joint applicant whose name appears first in alphabetical order will be deemed to be the common representative under paragraph 218(b) of the Patent Rules.
Regarding patents, if there are joint patentees and no agent was appointed at any time prior to the granting of the patent, the patentee who was authorized correspondent under the former Patent Rules at that the time the patent was granted is deemed to be the common representative under section 219 of the Patent Rules.
In other cases where there are joint applicants or joint patentees (such as when a patent agent is appointed and is the authorized correspondent under the former Patent Rules), there will be no common representative as of October 30, 2019.
Joint applicants or patentees may appoint a common representative by submitting a notice to the Commissioner that is signed by all of the other joint applicants or patentees (under paragraph 26(3)(a) of the Patent Rules). Note that until a common representative is appointed, the signatures of all applicants or patentees will be required to appoint a patent agent. The signatures of all applicants or patentees will also be required to revoke the appointment of a patent agent, unless the patent agent signs the notice of revocation.
Date modified: October 2019
Any appointment of a patent agent or associate patent agent that was made prior to October 30, 2019, in accordance with the former Patent Rules, remains in effect after October 30, 2019 and is considered to have been made in accordance with the Patent Rules (sections 216 and 217 of the Patent Rules (SOR/2019-251), see Chapter 5 for more information).
Date modified: October 2019
The provisions in the Patent Rules (SOR/2019-251) regarding representation for the purpose of procedures relating to a granted patent (section 37 of the Patent Rules (SOR/2019-251)) will not apply to any procedure that started prior to October 30, 2019. The former Patent Rules will continue to apply to such procedures (section 223 of the Patent Rules).
Date modified: October 2022
As of June 28, 2021, patent agents must reside in Canada. As of June 28, 2021, the appointment of any patent agent that is not a resident of Canada in respect of any patent application, patent or other business before the Office is revoked. If the non-resident patent agent appointed an associate patent agent who is a resident of Canada, that resident patent agent is deemed to be appointed as the patent agent in respect of that business.
As of June 28, 2021, a patent agent is defined as an individual who holds a patent agent licence or a patent agent in training licence. Firms are not considered to be patent agents. Therefore, as of the CIF date, in any case where a firm was previously appointed as the patent agent in respect of an application, patent or other business before the Office, all of the patent agents at the firm are deemed to be appointed as patent agent in respect of that business. If a firm was previously appointed as the associate patent agent by a patent agent who resides in Canada, all of the patent agents at the firm are deemed to be appointed as the associate patent agent in respect of that business. If a firm was previously appointed as the associate patent agent by a patent agent who is not a resident of Canada, all of the patent agents at the firm are deemed to be appointed as patent agent in respect of that business.
Date modified: October 2019
Requests for priority made in accordance with the former Patent Rules before October 30, 2019 remain valid after that date. A request for priority made for a Category 3 application may be made or corrected after October 30, 2019 within the time outlined in subsection section 195 of the Patent Rules (SOR/2019-251). The request can be made in the petition or any separate document. Please note that the Office strongly discourages the request from being made in the abstract, specification or drawings (sections 188 and 195 of the Patent Rules (SOR/2019-251)).
The requirement to provide a copy or access to the priority application does not apply to a request for priority made before October 30, 2019. Examiners will have the authority to request, by notice, that the applicant provide a copy or access in a digital library to the priority application if the examiner takes into account the priority application during examination. If such notice is sent, the applicant will have four months to provide a copy or access through a digital library (sections 196 and 215 of the Patent Rules (SOR/2019-251)).
Date modified: October 2022
Restoration of the right of priority is not available for Category 3 applications (section 78.5 of the Patent Act, https://www.wipo.int/pct/en/texts/reservations/res_incomp.html).
Restoration of the right of priority is available for patent applications that have a filing date that is after October 30, 2019.
Date modified: October 2019
Section 78.51 of the Patent Act specifies that if no payment was made for a due date that falls before October 30, 2019, section 73 of the former Patent Rules will apply, with respect to deemed abandonment and reinstatement.
Otherwise, if the maintenance fee is due after October 30, 2019 and it is not paid before that date, the amended section 27.1 of the Patent Act and the Patent Rules (SOR/2019-251) will apply.
Date modified: June 2023
The reinstatement regime in section 73(3) of the Patent Act and section 98 of the former Patent Rules as they read before October 30, 2019 continues to apply where an application is abandoned before October 30, 2019 or is abandoned for failing to do any act described in paragraph 73(1)(a), (b), (e) or (f) of the Act as it read before October 30, 2019 in respect of a requisition made or a notice given (section 78.51 and subsection 78.52(1) of the Patent Act).
Abandonment under section 132 of the Patent Rules (SOR/2019-251) does not apply to Category 3 applications. Section 203 of the Patent Rules (SOR/2019-251) will apply and for the purposes of subsection 73(2) of the Patent Act. A Category 3 application is deemed to be abandoned if:
The applicant does not make a request for continued examination of an application for a patent and pay the prescribed fee within 4 months of the date of the notice in accordance with subsection 85.1(3) of the Patent Rules (SOR/2019-251);
An applicant requesting reinstatement of an application following a failure to pay a maintenance fee or a failure to request examination that occurred prior to October 30, 2019 is not subject to the due care standard.
Section 73 of the Patent Act, as it read immediately before October 30, 2019, applies with respect to reinstatement of applications deemed abandoned in these circumstances.
Date modified: October 2019
Examination of patent applications in progress will continue after October 30, 2019.
Date modified: December 2025
Section 197 of the Patent Rules (SOR/2019-251) specifies the prescribed time in which to make a request for examination with respect to Category 3 applications:
Date modified: October 2019
Examiner requisitions sent during the transition period of October 30, 2019 will have the following due dates:
If abandonment occurs after October 30, 2019 from a requisition sent before October 30, 2019, then deemed abandonment and reinstatement will be under section 73 of the Patent Act as it read immediately before October 30, 2019 (subsection 78.52(1) of the Patent Act).
Date modified: October 2022
Notices of allowance under section 30 of the former Patent Rules sent before October 30, 2019 will have a six month due date even if that due date comes after October 30, 2019. As outlined in section 78.52 of the Patent Act, a failure to pay the final fee on or after October 30, 2019 requisitioned in the notice of allowance dated before October 30, 2019 will result in the application being deemed abandoned under paragraph 73(1)(f) of the Patent Act as it read immediately before October 30, 2019. The application can be reinstated in the 12 month period that follows the abandonment under section 73(3) of the Patent Act as it read immediately before October 30, 2019.
Amendments after allowance may be made under section 32 of the former Patent Rules where a notice of allowance was sent before October 30, 2019, except if the application were to have been abandoned for failure to pay the final fee under paragraph 73(1)(f) of the Patent Act as it read before October 30, 2019 and subsequently reinstated. Otherwise, amendments after allowance are not permitted for Category 3 applications, except to correct obvious errors.
Section 204 of the Patent Rules (SOR/2019-251) specifies that Category 3 applications that are abandoned under paragraph 73(1)(f) of the Patent Act as it read before October 30, 2019 and then subsequently reinstated may have their final fee refunded, upon a request received no more than 1 month after reinstatement, thereby returning the application to examination. The act of reinstatement alone, in this context, returns the application to examination. Such a return to examination is as described in the Patent Act as ready before October 30, 2019. No refund of the final fee is required for this action. Otherwise, any reinstatement for abandonment for non-payment of a final fee as requisitioned from a notice given after October 30, 2019, will proceed directly to patent grant.
In those cases of Category 3 applications as discussed above, where the final fee was not refunded and upon re-allowance, the Commissioner will not require payment in a notice of allowance or a conditional notice of allowance sent after the reinstatement of the application (subsection 204(b) of the Patent Rules). Additionally, the refund does not automatically trigger a return to examination of the application.
Date modified: October 2022
Section 78.55 of the Patent Act specifies that section 46 of the Patent Act, as it read immediately before October 30, 2019 of the amended Patent Act and the Patent Rules (SOR/2019-251) applies to maintenance fee due dates (not including the period of grace) before October 30, 2019. If the maintenance fee due date (not including the period of grace) is before October 30, 2019 and the maintenance fee is not paid on or before that due date, the patentee will have a 12-month grace period as per item 31 or 32 of Schedule II of the former Patent Rules as they read immediately before October 30, 2019.
Date modified: October 2019
The requirements contained in subparagraph 154(3)(a)(i) of the Patent Rules (SOR/2019-251) (i.e. a request that the rights of the applicant be reinstated with respect to the international application and a statement that the failure to enter national phase within the 30-month deadline was unintentional) do not apply to Category 3 applications. The requirements only apply to an international application entering national phase in Canada when the international filing date is on or after the October 30, 2019.
For example:
Priority Date: May 1, 2017
Filing Date: May 1, 2018
Request to Enter National Phase: November 4, 2019
The applicant is requesting entry into the national phase after the 30-month deadline. Since the international filing date of the application is May 1, 2018, the requirements in subparagraph 154(3)(a)(i) will not apply to the applicant who enters national phase in Canada past the 30-month deadline (section 234 of the Patent Rules (SOR/2019-251)).
If the right of priority is restored during the international phase, it will not be deemed restored in Canada under section 162 of the Patent Rules (SOR/2019-251) unless the international filing date is on or after October 30, 2019. Restoration of the right of priority under subsection 28.4(6) of the Patent Act and section 77 of the Patent Rules (SOR/2019-251) will not be available to applicants upon national phase entry if the international filing date is before October 30, 2019 (section 78.5 of the Patent Rules (SOR/2019-251)).
Date modified: October 2022
The excess claim fee applicable when requesting examination and when paying the final fee applies to applications for which examination was requested on or after October 3, 2022.
A request for continued examination is required under subsection 85.1(1) of the Patent Rules in respect of applications for which examination has been requested on or after October 3, 2022.
If an applicant requests continued examination under subsection 85.1(3) or (4) of the Patent Rules on or after October 3, 2022, the applicant will be required to make a subsequent request for continued examination after two examiner reports are issued.
A conditional notice of allowance under subsection 86(1.1) of the Patent Rules may be sent in respect of any application on or after October 3, 2022.
Date modified: October 2019
The Patent Cooperation Treaty (PCT) is a multilateral treaty among States that concluded negotiations in 1970 and entered into force on January 24, 1978. Canada became a PCT signatory on January 2, 1990, and became an International Searching and Preliminary Examination Authority under the PCT on July 26, 2004. According to the World Intellectual Property Organization (WIPO) “The PCT makes it possible to seek patent protection for an invention simultaneously in each of a large number of countries by filing an "international" patent application. Such an application may be filed by anyone who is a national or resident of a PCT Contracting State. It may generally be filed with the national patent office of the Contracting State of which the applicant is a national or resident or, at the applicant's option, with the International Bureau of WIPO in Geneva.
Once the patent application has been filed internationally through the PCT, applicants may enter the national phase in the contracting states for which the applicant seeks patent protection.”[409]
Date modified: October 2022
An applicant who designates Canada in an international application must meet the requirements outlined in subsection 154(1) of the Patent Rules to enter the national phase not later than thirty months after the earliest priority date. If the requirements are not met by the 30-month deadline, an applicant may still enter national phase within 12 months after the 30-month deadline when additional requirements are met.
The requirements to enter the PCT national phase within 30 months after the priority date are as follows:
Applicants should consult the PCT Applicant’s Guide – National Phase as well as the Canadian National Chapter for further details and guidance.
Date modified: October 2022
When calculating deadlines to enter national phase in Canada, “priority date” has the same meaning as in Article 2(xi) of the Patent Cooperation Treaty. For the purposes of calculating time limits, the priority date is the filing date of the earliest application whose priority is claimed, or the filing date of the international application when there is no priority claim.
The priority date may only change in one of the following three scenarios during the international phase:
It is important to note that even if the filing date of the international application is more than 12 months after the filing date of the application whose priority is claimed, and if the priority is not restored (either in the international phase or the national phase), the priority date will still be used to calculate the time limit to enter national phase.
Date modified: October 2022
If a PCT international phase application does not enter the national phase in Canada within 30 months after the priority date, the applicant has 12 months after that time to reinstate the rights of the applicant to enter the national phase with respect to the international application under subsection 154(3) of the Patent Rules.
The requirements to reinstate the rights of the applicant for PCT national phase entry are as follows:
Date modified: October 2022
If, after the 30-month time limit but within 12 months after that time limit, the Commissioner receives a communication clearly indicating the applicant’s intention to pay some or all of the fees required to enter the national phase, but not all of the required fees are paid within that period, those fees are considered to have been paid on the day the communication was received as long as all unpaid fees – including applicable late fees – are paid within two months after the communication was received. The Commissioner does not have an obligation to inform the person who attempted to pay the fees that part of the required fees were missing. However, the Office will endeavour to inform the applicant of the missing fees by a courtesy letter.
Date modified: October 2022
An application may enter national phase with elements of the application in a foreign language. However, if a translation of those elements, or any part of those elements are not provided on or before the national phase entry date, various consequences may arise depending on the element of the application that is missing a translation.
The applicant must also provide a complete copy of the element of the application that contains the translated text matter and the text matter that is already in English or French. If a complete copy is provided, the applicant does not have to provide translations of the parts that are not in English or French.
If the applicant does not provide translations of the parts of the description or claims, or the text matter in a drawing or sequence listing, the untranslated text will not be taken into account for the purpose of interpreting the scope of protection being sought (see 12.02).
If the applicant does not provide a translation of an Article 19 statement, the statement may be disregarded by the Commissioner.
Date modified: October 2022
If the applicant does not provide a translation of the abstract or the article 4 PCT request (RO/101), or if the applicant does not provide a complete copy of the description, claims, text matter in a drawing, or language-dependent free text contained in a sequence listing, the Commissioner may by notice require the applicant to provide the translation of the complete copy no later than three months after the date of the notice.
If the applicant does not comply with the notice, the application will be deemed to be abandoned under subsection 132(1)(h) of the Patent Rules.
Date modified: October 2022
If an applicant discovers an error in a translation that was submitted to the Commissioner on or before the national phase entry date of any element of an international application, the applicant may, under subsection 155.2(2) of the Patent Rules, correct the translation by submitting the following before the day on which a notice of allowance or a conditional notice of allowance is sent or, if that notice is withdrawn by the Commissioner or set aside in accordance with subsection 85.1(4) of the Patent Rules, before the day on which a notice of allowance or a conditional notice of allowance is sent again:
Date modified: October 2019
The national phase entry date of an application is the date on which the applicant either:
When a national phase entry date is established, the Patent Office will send the applicant an acknowledgement of national phase entry.
Date modified: September 2020
If an international application is published in English or French by the International Bureau on or before its national phase entry date, the PCT national phase application is considered to be open to public inspection under section 10 of the Patent Act on the date of that publication.
If an international application is published in a language other than English or French by the International Bureau on or before its national phase entry date, the PCT national phase application is considered to be open to public inspection when the application is made publicly available in Canada.
Date modified: October 2022
If the Commissioner has reasonable grounds to believe that the person who met the requirements to enter national phase is neither the applicant of the international application nor their legal representative, the Commissioner must send a notice under subsection 154(7) of the Patent Rules requiring that person to establish that they are either the applicant of the international application or their legal representative.
The person may establish that they are either the applicant of the PCT international application or their legal representative by providing to the Patent Office a PCT/IB/306 form showing a change in the applicant of the international application, a document effecting a transfer to the person who complied with the requirements to enter national phase in Canada, or a change of name document. The Office may consider other submissions acceptable, if necessary. The Patent Office will not accept a copy of a request made to the International Bureau under section 92bis.1 of the Regulations under the Patent Cooperation Treaty as acceptable means to establish applicant rights in response to a requisition under subsection 154(7) of the Patent Rules.
A person may also respond to the notice by submitting a compliant request under subsection 154(6) of the Patent Rules to correct an error in the naming of the applicant who complied with the requirements to enter national phase. If the request for correction is compliant and results in the name of the person who complied with the requirements to enter national phase being the same as the applicant of the international application, the person will be considered to have complied with the notice.
Pursuant to subsection 154(8) of the Patent Rules, where the person does not comply with the notice within three months after the date of the notice, that person is deemed never to have complied with the requirements to enter the national phase in Canada.
Date modified: October 2022
If Patent Office records contain an error in the naming of the applicant who complied with the requirements to enter national phase, the error may be corrected on the request of the person who paid the basic national fee. It should be noted that the person who paid the fee is considered the person who submitted the fees, not necessarily the applicant. The request must contain a statement that the error arose inadvertently, or by accident or mistake, and without any fraudulent or deceptive intention.
The request to correct must be made before the earlier of:
and
A correction to the naming of the applicant who met the requirements to enter national phase may result in a discrepancy between the applicant who met the requirements to enter national phase and the applicant of the international application. If this discrepancy results from the correction, a notice under subsection 154(7) may be sent to the applicant.
Date modified: October 2022
In general, a PCT national phase application is subject to Canada’s Patent Act and Patent Rules on the national phase entry date. However, there are certain exceptions and the following sections of the Patent Act do not apply to PCT national phase applications:
It should also be noted that certain administrative elements and requirements in the Patent Rules are specific to PCT national phase applications, such as the manner of appointment of a patent agent, the deemed appointment of the common representative, requirements relating to requests for priority and restoration of the right of priority, and certain corrections of an error in the name of the applicant (described in Section 34.06 above). Please see the relevant Chapters for more information.
Date modified: October 2022
Section 78 of the Patent Act does not apply to international applications during the international phase. During the international phase, extensions of time are available only as provided for under the PCT and the Regulations under the PCT, including under PCT Rules 80.5, 82 and 82quater. Detailed information about extensions of time during the international phase is available in the PCT Applicant’s Guide.
Date modified: October 2019
Section 161 of the Patent Rules states the filing date of a PCT national phase application is the international filing date.
Date modified: October 2022
A request for the restoration of the right of priority is a mechanism used by the receiving Office of the International Bureau and the receiving offices of numerous PCT signatory countries, whereby the time limit for filing an application may be extended beyond the normal 12-month period after the filing date of a priority document. This practice is limited to situations where the applicant failed to file a request for priority despite due care and/or where the failure to request was unintentional on the part of the applicant.
When acting as a receiving office for international applications, CIPO will accept a request by the applicant to restore the right of priority for an international application if it is satisfied that the criteria are met. This restoration may then be effective in designated offices whose applicable national laws provide for the restoration of the right of priority.
For PCT national phase applications with filing dates on or after October 30, 2019, restoration of the right of priority that occurred prior to national phase entry will be deemed restored in Canada upon national phase entry. Applicants may also request restoration of the right of priority upon entry into the national phase in Canada where the filing date of the PCT national phase application is on or after October 30, 2019. For regular patent applications filed in Canada and for PCT national phase applications, applicants can request the restoration of the right of priority when the filing date of the pending application is more than twelve months after the filing date of the previously regularly filed application, but within two months after the end of those twelve months. See Chapter 7 for more information.
Date modified: October 2019
Applicants are not required to send a petition to the Patent Office for entry into the national phase in Canada
The National Chapter of the PCT Applicant’s Guide for national phase entry in Canada contains a form that applicants may use to submit national entry requirements and other information. The form is entitled “Recommended Form for request of Entry into National Phase under Article 22/Article 39 of the Patent Cooperation Treaty”.
WIPO provides an abundance of online resources covering the Patent Cooperation Treaty (PCT). The main webpage can be found here: PCT – The International Patent System.
The PCT Applicant’s Guide is separated into a guide with general information on the international phase, as well as a guide with general information on the national phase.
The PCT Applicant’s Guide also contains information for applicants wishing to file an international application with CIPO and for applicants who wish to enter the national phase in Canada. Information relating to CIPO as a receiving Office, an International Search Authority and an International Preliminary Examination Authority can also be found in the PCT Applicant’s Guide’s Canadian annexes.
The relevant parts of the PCT Applicant’s Guide referred to above can be found here:
Links to the PCT Treaty, Regulations and Administrative Instructions can be found here:
CIPO follows WIPO’s Guidelines for Authorities and Offices.
Date modified: October 2019
All business related to patent applications and patents before the Patent Office is done in writing[410]: both that which is submitted to the Office or sent from the Office. This chapter is a guide to notices, letters and requisitions sent from the Office, which encompasses most of the correspondence issued by the Office.
Date modified: October 2019
Various provisions of the Patent Act and the Patent Rules require the Commissioner to notify an applicant or patentee that an action is required to be taken. Commissioner’s notices require the applicant or patentee to perform an action on or within a time period after the date of the notice. Failure to take the action within the time period will lead to a consequence identified in the Patent Act or the Patent Rules.
Example 1:
An applicant submits documents to the Office that meet the minimum requirements to obtain a filing date under subsection 28(1) of the Patent Act but did not submit the application fee required under subsection 27(2) of the Patent Act. The Commissioner will send a notice under subsection 27(7) of the Patent Act to pay the application fee and the late fee within three months of the date of the notice. Failure to do so within those three months will result in the application being considered withdrawn under subsection 66(2) of the Patent Rules.
Example 2:
An applicant has requested priority in respect of a pending application and is required to either submit a copy or make a copy available in a digital library of that priority application under subsection 74(1) of the Patent Rules within a specific time period. If the applicant has not submitted or made available a copy within the specified time, the Commissioner will send a notice under subsection 74(4) of the Patent Rules requiring the applicant to submit or make available the copy within two months of the date of the notice. Failure to do this within those two months will result in the request for priority being considered to have been withdrawn under subsection 74(6) of the Patent Rules.
Example 3:
Annual maintenance fees are required to maintain an application in effect (subsection 27.1(1) of the Patent Act and sections 68 and 69 of the Patent Rules). If the maintenance fee is not paid on or before its due date, the Commissioner will send a notice to the applicant under paragraph 27.1(2)(b) of the Patent Act, requiring the applicant to pay the maintenance fee and the late fee before the later of six months from the maintenance fee due date or two months after the date of the notice. Failure to do so will result in the application being deemed abandoned under paragraph 73(1)(c) of the Patent Act.
Date modified: October 2021
Commissioner’s notices are generally structured in a bilingual format and are intended to provide applicants and patentees with key pieces of information to easily identify the application or patent to which the notice relates and to identify the action that is required on the part of the applicant or patentee.

The following describes details typically found in Commissioner’s notices:
Header: A Commissioner’s notice will be identified as such in the header and identify the reason for the notice in plain language.
Notice Details:
General Information on the Application or Patent:
Text in Body of Notice:
Closing Paragraph:
Date modified: December 2025
The following is a non-exhaustive list of Commissioner’s notices that can be sent under the Patent Act and the Patent Rules as well as their legislative references:
Patent Rules
·Section 65 Notice of Non-Compliance
·Subsection 72(1) Notice of Missing Parts
·Subsection 74(4) Copy of Priority Document Required
·Subsections 85.1(1) and 85.1(2) Notice Requiring Continued Examination (this notice will likely be contained in an examiner’s report (see section 35.04 below))
·Subsections 86(1), (6), (10), (12) Notice of Allowance
·Subsection 86(1.1) Conditional Notice of Allowance
·Subsections 86(14) Notice of Withdrawal of Notice of Allowance
·Subsections 86(14.1), (15) Notice of Withdrawal of Conditional Notice of Allowance
·Subsection 109(3) Missing Information or Fee for Correction of an Error in a Patent
·Subsection 154(7) PCT Applicant Entitlement Notice
·Subsection 155.2(1) PCT Notice of Error in Translation
·Subsection 155.5(6) PCT Compliance Notice for Translation
Date modified: October 2022
Prosecuting and maintaining patent applications and patents typically involves numerous written exchanges with the Office and payments of fees. The Office aims to respond to clients in a clear and timely manner and to be transparent on how client submissions have been processed in the Office, so that clients can make informed decisions on their patent application or patent.
In most cases[411], the Office will inform clients via a courtesy letter that a communication and/or a fee has been received and whether it is compliant or not compliant with the Patent Act and the Patent Rules. The Office will also aim to inform clients if their applications are deemed abandoned, including the reason, or if their patents are deemed expired for failure to pay a maintenance fee and the late fee.
Examples of courtesy letters:
Date modified: October 2019
Courtesy letters are generally structured in a bilingual format and are intended to provide applicants and patentees the key pieces of information needed.

The following describes details typically found in courtesy letters:
Header: A courtesy letter will be identified as such in the header and identify in plain language the reason for the letter.
Notice Details:
General Information on the Application or Patent:
Text in Body of Letter:
Closing Paragraph:
Date modified: October 2022
Examiner reports and notices take a different form than notices from the Commissioner. An examiner’s report or notice is unilingual and the date is located in the top corner of the document. Each examiner’s report or notice includes an opening paragraph that indicates the required time period for response for each requisition and may inform the applicant of a requirement to request for continued examination within a required time period. The consequence for non-response within that time is identified in the opening or closing paragraph of the requisition or notice. The due date for each requisition does not appear on the requisition or notice. The applicant can calculate the due date using the time period specified in the opening paragraph and adding it to the date provided on the notice or requisition. If the time period ends on a prescribed or designated day, the time period is deemed extended under subsection 78(1) of the Patent Act. For more information on time limits and deemed extensions of time, please Chapter 2. More information on examiner reports and notices can be found in sections 12.04 and 12.05.
Date modified: October 2022
The conditional notice of allowance also takes a different form than other notices from the Commissioner. The first page of the conditional notice of allowance will be bilingual like other notices from the Commissioner The due date to response to the conditional notice of allowance as well as the fee owing will be indicated in the top right corner of the notice. The defects listed in the conditional notice of allowance will be on the second page and unilingual. The notice will include the consequence of not responding to the conditional notice of allowance and not paying the fee (abandonment). For more information on time limits and deemed extensions of time, please see Chapter 2. More information on conditional notice of allowance can be found in section 25.01.01.
[1] PCT sequence listing standard is defined in subsection 1(1) of the Patent Rules to mean Standard ST.26 of the World Intellectual Property Organization, Recommended Standard for the Presentation of Nucleotide and Amino Acid Sequence Listings using XML (eXtensible Markup Language), as amended from time to time.
[3] Alphabetical order will be based on surnames, and then on first given name, both using the Roman alphabet. Entries beginning with numbers are positioned before those beginning with letters.
[4] Exception: A request for advanced examination on environmental grounds must be made by the single applicant or common representative.
[5] Proof of authorization (by any applicant) required
[6] When a request to record the transfer of an application is made by the transferee, the transferee may represent themselves or be represented by any person authorized by them.
[7] If the rights of only one joint applicant who is not the common representative are being transferred, that joint applicant may also submit the request or authorize another person to do so.
[8] Exception: A request for advanced examination on environmental grounds must be made by the appointed agent.
[9] Proof of authorization (by any applicant) required.
[10] When a request to record the transfer of an application is made by the transferee, the transferee may represent themselves or be represented by any person authorized by them.
[11] If the rights of only one joint applicant who is not the common representative are being transferred, that joint applicant may also submit the request or authorize another person to do so.
[12] Requires permission of appointed agent.
[13] Proof of authorization (by single applicant or common representative) required.
[14] Proof of authorization (by any patentee) required.
[15] When a request to record the transfer of an application is made by the transferee, the transferee may represent themselves or be represented by any person authorized by them.
[16] If the rights of only one joint patentee who is not the common representative are being transferred, that joint patentee may also submit the request or authorize another person to do so.
[17] The form will be developed and posted at a later date.
[18] Pfizer Canada v. Ratiopharm Inc. 2010 FC 612 at paragraph 84, referring to Apotex Inc.v. Merck & Co. 2006 FCA 323, [2007] 3 F.C.R. 588 at paragraph 55.
[19] “Regularly filed application” means any application which bears as its filing date the date on which it is received by the Office or an application filed in the Office at the national stage of an international application.
[20] Other recognised intergovernmental authorities include the Eurasian Patent Organization (EAPO) and the Gulf Cooperation Council Patent Office (GCCPO).
[21] See Article 11(4) of the PCT.
[22] The European Patent Office (EPO) grants patents enforceable in any Contracting State of the European Patent Convention (EPC) [see Article 2(2) of the EPC] unless the applicant for the European patent has withdrawn a Contracting State from designation [see Article 79(3) of the EPC]; a granted patent must, however, be validated in each Contracting State.
[23] The term inventors’ certificate replaces the formerly used authors’ certificate but has the same effect. The change was made in the Paris Convention to avoid confusion with copyright authorship.
[24] See Article 4(I)(2) of the Paris Convention.
[25] Presentation date of the divisional application is the date on which the filing requirements for a divisional application have been met.
[26] This does not apply to applications for which the request for examination was made prior to October 3, 2022.
[27] This does not apply to applications for which the request for examination was made prior to October 3, 2022.
[28] This does not apply to applications for which the request for examination was made prior to October 3, 2022 unless there was a request for continued examination made under subsection 85.1(4) of the Patent Rules (see section 11.04.02c) on or after October 3, 2022.
[29] This does not apply to applications for which the request for examination was made prior to October 3, 2022 unless there was a request for continued examination made under subsection 85.1(4) of the Patent Rules (see section 11.04.02c) on or after October 3, 2022.
[31] Purposive construction is performed by the court to objectively determine what the person skilled in the art would, as of the date of publication of the patent application and on the basis of the particular words or phrases used in the claim, have understood the applicant to have intended to be the scope of protection sought for the disclosed invention (see Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 50; and Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at paragraph 48).
Free World Trust and Whirlpool continue to guide the courts, with the benefit of expert testimony and cross-examination, to construe the claim in accordance with the principles of purposive construction outlined therein. (For an enumeration of the principles, see Free World Trust at paragraph 31).
However, Whirlpool was an impeachment proceeding that was not directed “to patent examiners in the course of examinations to determine whether applications for patents should be granted.” (see Genencor International Inc. v. Canada (Commissioner of Patents), 2008 FC 608 [Genencor] at paragraphs 62 and 70).
It should be recognized that the language of patent claims construed by judges is fixed, is the result of a negotiation with the Patent Office, was “accepted by the Commissioner of Patents as a correct statement of a monopoly that can properly be derived from the invention disclosed in the specification”
(see Whirlpool at paragraph 49) and benefits from the presumption of validity accorded by subsection 43(2) of the Patent Act. In contrast, during examination of an application the language of the claim may change from that initially proposed by the applicant for a number of reasons (see Genencor at paragraphs 62 and 70 and Amazon FCA at paragraph 73).
[32] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 50; Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at paragraph 48
[33] More information on free text and language-dependent free text can be found in paragraphs 85-88 of the PCT sequence listing standard, WIPO Standard 26 (ST.26).
[34] Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at paragraphs 49(f)(g), 52 and 53
[35] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 51
[36] Canada (Attorney General) v. Amazon.com Inc., 2011 FCA 328 at paragraph 73
[37] Merck & Co., Inc. v. Pharmascience Inc. 2010 FC 510 at paragraphs 32 and 35
[38] Bayer Aktiengesellschaft v. Apotex Inc. [(1995), 60 C.P.R. (3rd), 58 (On.Ct.G.D.)] at page 79; Johnson & Johnson Inc. v. Boston Scientific Ltd. 2008 FC 552 at paragraph 97; Lundbeck Canada Inc v. Minister of Health 2009 FC 146 at paragraph 36; Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 at paragraph 122
[39] From Beloit Canada Ltd. v. Valmet Oy [(1986), 8 C.P.R. (3rd), 289 (F.C.A.)] at page 294 we know them to be a paragon of deduction. See also the comments on point in Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraph 113.
[40] Bayer Aktiengesellschaft v. Apotex Inc. [(1995), 60 C.P.R. (3rd), 58 (On.Ct.G.D.)] at page 79; Merck-Frosst - Schering Pharma GP v. Teva Canada Limited 2010 FC 933 at paragraphs 68 and 69
[41] Servier Canada Inc. v. Apotex Inc. 2008 FC 825 at paragraph 99; Lundbeck Canada Inc. v. Ratiopharm Inc. 2009 FC 1102 at paragraph 29; Sanofi-Aventis Canada Inc. v. Apotex 2009 FC 676 at paragraph 80
[42] Almecon Industries Ltd. v. Nutron Manufacturing Ltd. (1997) 72 C.P.R. 3d 397 at page 401.
[43] Whirlpool Corp. v. Camco Inc. 2000 SCC 67 at paragraph 74; Servier Canada Inc. v. Apotex Inc. 2008 FC 825 at paragraph 254; Newco Tank Corp v. Canada (Attorney General) 2014 FC 287 at paragraph 28.
[44] Axcan Pharma Inc. v. Pharmascience Inc., 2006 FC 527 at paragraph 38
[45] Servier Canada Inc. v. Apotex Inc. 2008 FC 825 at paragraph 236
[46] Ratiopharm Inc. v. Pfizer Limited 2009 FC 711, at paragraph 30, aff’d 2010 FCA 204
[47] GlaxoSmithKline Inc. v. Pharmascience Inc., 2008 FC 593 at paragraph 35
[48]. Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraph 90.
[49] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 at paragraph 121
[50] Merck & Co., Inc. v. Pharmascience Inc. 2010 FC 510 at paragraph 40; AstraZeneca Canada Inc. v. Apotex Inc. 2010 FC 714 at paragraph 39
[51] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 37
[52] Sanofi-Aventis Canada Inc. v. Apotex 2009 FC 676 at paragraph 304
[53] Whirlpool Corp. v. Camco Inc. 2000 SCC 67 at paragraph 74
[54] Eli Lilly and Company v. Apotex Inc. 2009 FC 991 at paragraph 97, citing General Tire & Rubber Co. v. Firestone Tyre & Rubber Co. Ltd, [1972] RPC 457 at pages 482-483
[55] Eli Lilly and Company v. Apotex Inc. 2009 FC 991 at paragraph 421
[56] Shire Biochem Inc. v. Minister of Health 2008 FC 538 at paragraph 25; Eli Lilly Canada Inc. v. Novopharm Ltd. 2007 FC 596 at paragraph 142; Pfizer Canada Inc. v. Novopharm Ltd. 2005 FC 1299 at paragraph 78; Whirlpool Corp. v. Camco Inc. [(1997), 76 C.P.R. (3rd), 150 (F.C.T.D.)] at page 186
[57] Apotex Inc. v. Wellcome Foundation Ltd., 2002 SCC 77 at paragraph 37; the Supreme Court in Teva Canada Ltd. v. Pfizer Canada Inc., 2012 SCC 60 at paragraph 32 reiterates this point, and speaks of the importance of the patent bargain in advancing science and technology.
[58] AstraZeneca Canada Inc. v. Apotex Inc., 2010 FC 714 at paragraph 33; Wenzel Downhole Tools Ltd. v National-Oilwell Canada Ltd., 2011 FC 1323 at paragraph 61; Jay-Lor International Inc. v. Penta Farm Systems Ltd., 2007 FC 358 at paragraph 55; Sanofi-Aventis Canada Inc. v. Apotex, 2009 FC 676 at paragraph 128; Merck & Co. Inc. v. Apotex Inc., 2010 FC 1265 at paragraph 86
[59] Canada (Attorney General) v. Amazon.com Inc., 2011 FCA 328 at paragraph 43
[60] Canada (Attorney General) v. Amazon.com Inc., 2011 FCA 328 at paragraph 42. It is also stated that the examiner must be “alive to the possibility that a patent claim may be expressed in language that is deliberately or inadvertently deceptive”
, thus recognizing that, “for example, what appears on its face to be a claim for an “art” or a “process” may, on a proper construction, be a claim for a mathematical formula and therefore not patentable subject matter”
(see Amazon FCA at paragraph 44).
[61] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 58
[62] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 55
[63] Canada (Attorney General) v. Amazon.com Inc., 2011 FCA 328 at paragraphs 59 to 63; following the reasoning of the court, the existence of a practical embodiment does not automatically imply that the elements of the embodiment are essential elements of the invention.
[64] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at paragraph 52
[65] Halford v Seed Hawk Inc., 2006 FCA 275 at paragraph 14
[66] The Office does not consider the “self-inflicted wound” factor to be relevant during examination.
[67] Examiners should be mindful that, in this context, the identification of multiple problems and solutions within a single claim is not to be confused with lack of unity of invention within the meaning of section 88 of the Patent Rules (which emphasizes that the subject matter defined by the claims are to be linked by a single general inventive concept).
[68] Re Application for Patent of Prince Corp., 1982, 2 C.P.R. (3d) 223 (CD 942); and Shmuel Hershkovitz v. Tyco Safety Products Canada Ltd., 2009 FC 256 at paragraph 148
[69] The “claim date” of a claim in an application or patent is the filing date of the application in Canada, unless there is a priority claimed. In the latter case the claim date is the filing date of the earliest priority application which supports the subject matter of the claim.
[70] Searches performed by Canadian examiners as part of CIPO’s obligations as an International Searching Authority are governed by the requirements of the PCT, and are not covered by this section of the manual.
[71] See subsection 27(4) of the Patent Act and the definition of: “description” in subsection 1(1) of the Patent Rules.
[73] Pioneer Hi-Bred Ltd. v. Canada (Commissioner of Patents) [(1989), 25 C.P.R. (3rd), 257 (S.C.C.)] at p268; Apotex Inc. v. Wellcome Foundation Ltd., 2002 SCC 77 at para 70; Electrolytic Zinc Process Co. v. French’s Complex Ore Reduction Co., [1930] S.C.R. 462 at para 22; Leithiser v. Pengo Hydra-Pull of Canada Ltd. [(1974), 17 C.P.R. (2nd), 110 (F.C.A.)] at p113-115; Lundbeck Canada Inc. v. Minister of Health, 2009 FC 146 at para 135; Pfizer Canada Inc. v. Novopharm Limited, 2009 FC 638 at para 105. See also Apotex Inc. v. Sanofi-Synthelabo Canada Inc., 2008 SCC 61, e.g. at para 26, applying these requirements to prior disclosures being considered for the purposes of anticipation.
[74] Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at p154-155, Dickson J. quoting H.G. Fox from his Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed.]
[75] Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at p157
[76] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1947), 12 C.P.R. (1st), 102 (Ex.Ct.)] at p111
[77] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1947), 12 C.P.R. (1st), 102 (Ex.Ct.)] at p111
[78] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1947), 12 C.P.R. (1st), 102 (Ex.Ct.)] at p111-112, with these points being reasserted by Thurlow J. in Société des Usines Chimiques Rhone-Poulenc et al. v. Jules R. Gilbert Ltd. et al. [(1968), 55 C.P.R. (1st), 207 (S.C.C.)] at p225-226; Wandscheer et al. v. Sicard Limitée [(1947), 8 C.P.R. (1st), 35 (S.C.C.)] at p39-40.
[79] This position has been adopted by the courts so often that it has become axiomatic. See, e.g., Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at para 53; Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at p160
[80] Free World Trust v. Électro Santé Inc., 2000 SCC 66 at para 44, quoting H.G. Fox from his Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed.] at page 184; Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at para 49, citing Lister v. Norton Brothers and Co. [(1986), 3 R.P.C. 199 (Ch.D.)] at p203
[82] From Beloit Canada Ltd. v. Valmet Oy [(1986), 8 C.P.R. (3rd), 289 (F.C.A.)] at p294 we know them to be a “paragon of deduction” and from Whirlpool Corp. v. Camco Inc., 2000 SCC 67 at para 74 we know them to be “reasonably diligent in keeping up with advances in the field to which the patent relates”. See also the comments on point in Janssen-Ortho Inc. v. Novopharm Limited, 2006 FC 1234 at para 113.
[83] Bayer Aktiengesellschaft v. Apotex Inc. [(1995), 60 C.P.R. (3rd), 58 (On.Ct.G.D.)] at page 79
[85] Servier Canada Inc. v. Apotex Inc., 2008 FC 825 at para 254
[87] Bayer Aktiengesellschaft v. Apotex Inc. [(1995), 60 C.P.R. (3rd), 58 (On.Ct.G.D.)] at p79; Johnson & Johnson Inc. v. Boston Scientific Ltd., 2008 FC 552 at para 97; Lundbeck Canada Inc v. Minister of Health, 2009 FC 146 at para 36
[88] The comments in GlaxoSmithKline Inc. v. Pharmascience Inc., 2008 FC 593 at para 35, while they relate to expert witnesses at trial and not to examiners and inventors/applicants during examination, are illustrative.
[89] see, e.g., Apotex Inc. v. Sanofi-Synthelabo Canada Inc., 2008 SCC 61 at para 37; Burton Parsons Chemical Inc. v. Hewlett-Packard (Canada) Ltd. [(1976), 17 C.P.R. (2nd), 97 (S.C.C.)] at p105
[90] Pfizer Canada Inc. v. Novopharm Limited, 2009 FC 638 at para 108; Sanofi-Aventis Canada Inc. v. Apotex Inc., 2009 FC 676 at para 233; Free World Trust v. Électro Santé Inc., 2000 SCC 66 at para 54. Note, however, that the Supreme Court in Free World Trust was addressing the date for claim construction rather than enablement.
[91] Apotex Inc. v. Sanofi-Synthelabo Canada Inc., 2008 SCC 61 at para 37. During examination, such obvious errors should be corrected whenever identified.
[92] TRW Inc. v. Walbar of Canada Inc. [(1991), 39 C.P.R. (3rd), 176 (F.C.A.)] at p197
[93] Procter & Gamble Co. v. Bristol-Myers Canada Ltd. [(1978), 39 C.P.R. (2nd), 145 (F.C.T.D.)] at p159-160, aff’d [(1979), 42 C.P.R. (2nd), 33 (F.C.A.)]; see also Apotex Inc. v. Sanofi-Synthelabo Canada Inc., 2008 SCC 61 at paras 33-37
[94] Rice v. Christiani & Nielsen [1929] Ex.C.R. 111 at para 9, rev’d on other grounds
[95] H.G. Fox, Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed., Carswell (Toronto)] at p171; the last sentence in the first paragraph was quoted with approval in Pioneer Hi-Bred Ltd. v. Canada (Commissioner of Patents) [(1989), 25 C.P.R. (3rd), 257 (S.C.C.)] at p270
[96] Janssen-Ortho Inc. v. Novopharm Ltd., 2004 FC 1631 at para 54; quoted in Bristol-Myers Squibb Canada Co. v. Novopharm Ltd., 2005 FC 1458 at para 71, Aventis Pharma Inc. v. Apotex Inc., 2005 FC 1504 at para 126. Note that in the foregoing cases the Courts were addressing the question of obviousness, and whether engaging in routine testing made the result of that testing unobvious. However, the link between the obviousness analysis and the evaluation of sufficiency is addressed in Sanofi-Aventis Canada Inc. v. Ratiopharm Inc., 2010 FC 230 at paras 57-80. See also the comments in Pfizer Limited v. Ratiopharm, 2010 FCA 204 at paras 16 to 27.
[98] Janssen-Ortho Inc. v. Apotex Inc., 2008 FC 744 at para 111; Pfizer Canada Inc. v. Canada (Minister of Health), 2006 FCA 214 at paras 26 and 27
[102] Norac Systems International Inc. v. Prairie Systems & Equipment Ltd., 2002 FCT 337 at para 16, rev’d in part on other grounds 2003 FCA 187
[103] Dimplex North America Ltd. v. CFM Corp., 2006 FC 586 at para 80, aff’d 2007 FCA 278; citing Norac Systems International Inc. v. Prairie Systems & Equipment Ltd., 2002 FCT 337
[104] H.G. Fox, Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed., Carswell (Toronto)] citing at p150-151 Mullard Radio Valve Company Ltd. v.Philco Radio and Television Corporation of Great Britain Ltd. [(1935), 52 R.P.C. 261] at p287; quoted in Eli Lilly Canada Inc. v. Novopharm Ltd., 2007 FC 596 at para 188 and in Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1978), 39 C.P.R. (2nd), 191 F.C.T.D.)] at p216
[105] Norac Systems International Inc. v. Prairie Systems & Equipment Ltd., 2002 FCT 337 at para 41; Almecon Industries Ltd. v. Anchortek Ltd., 2001 FCT 1404 at para 45, aff’d 2003 FCA 168, citing Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1978), 39 C.P.R. (2nd), 191 F.C.T.D.)] at p216
[106] Metalliflex Ltd. v. Rodi & Wienenberger Aktiengesellschaft [(1960), 35 C.P.R. (1st), 49 (S.C.C.)] at p53-54
[107] see, e.g., Novopharm Limited v. Janssen-Ortho Inc., 2007 FCA 217 at para 26; Johnson & Johnson Inc. v. Boston Scientific Ltd., 2008 FC 552 at paras 376-377; Pfizer Canada Inc. v. The Minister of Health, 2008 FC 13 at paras 99 and 118
[108] The King v. American Optical Co. [(1950), 13 C.P.R. (1st), 87 (Ex.Ct.)] at p98
[109] The King v. American Optical Co. [(1950), 13 C.P.R. (1st), 87 (Ex.Ct.)] at p98
[110] Lester v. Commissioner of Patents [(1946), 6 C.P.R. (1st), 2 (Ex.Ct.)] citing at p3 British Celanese Ltd. v. Courtaulds Ltd. [1935] 52 R.P.C. 171 at p193
[111] Domtar Ltd. v. MacMillan Bloedel Packaging Ltd. [(1977), 33 C.P.R. (2nd), 182 (F.C.T.D.)] at p189-190; Bergeon v. De Kermor Electric Heating Co. [1927] Ex. C.R. 181 at paras 29 and 81; Schmuel Hershkovitz v. Tyco Safety Products Canada Ltd., 2009 FC 256 at para 148; Free World Trust v. Électro Santé Inc., 2000 SCC 66 at para 27
[113] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1952), 15 C.P.R. (1st), 133 (P.C.)] at p144-145
[114] This practice was first communicated in the practice notice Title of Invention [C.P.O.R. Vol. 137, No. 4, January 27, 2009].
[115] This requirement is explicitly governed by subsection 51(1) of the Patent Rules.
[116] The permissibility of chemical and mathematical formulae is provided by subsection 51(2) of the Patent Rules.
[117] The permissibility of such presentation in applications is implied from subsection 51(2) of the Patent Rules.
[118] Information regarding the publication of US patent documents is provided based on an interpretation of US practice as expressed in the USPTO’s Manual of Patent Examining Procedure, 8th Ed. (August 2001) as revised July 2008. See, e.g., sections 101 and 103.
[119] Natural Colour Kinematograph Co. v. Bioschemes Ltd. 32 R.P.C. 256 at page 266; this passage also cited in Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1952), 15 C.P.R. (1st), 133 (P.C.)]
[120] Any such amendment, of course, must not introduce new subject-matter such as to contravene section 38.2 of the Patent Act.
[121] Natural Colour Kinematograph Co. v. Bioschemes Ltd. 32 R.P.C. 256 at page 266. The use of “ambiguous” in this context should be understood in the context of the entire passage, wherein it was earlier stated that a patent is invalid if it relies on “language which, when fairly read, is avoidably obscure or ambiguous”
.
[122] Shell Oil v. Commissioner of Patents [(1982), 67 C.P.R. (2nd), 1 (S.C.C.)] at pages 10-11
[123] Canadian Gypsum Co. Ltd. v. Gypsum, Lime & Alabastine, Canada, Ltd. [1931] Ex.C.R. 180
[124] Tennessee Eastman v. Commissioner of Patents [(1972), 8 C.P.R. (2nd), 202 (S.C.C.)]
[125] Shell Oil v. Commissioner of Patents [(1982), 67 C.P.R. (2nd), 1 (S.C.C.)] at pages 10-11
[126] Commissioner of Patents v. Ciba Ltd. [(1959), 30 C.P.R. (1st), 135 (S.C.C.)] at page 141; aff’g [(1957), 27 C.P.R. (1st), 82 (Ex.Ct.)]
[127] "machine noun" The Oxford Dictionary of English (revised edition), Oxford University Press 2005; "machine" The Concise Oxford Dictionary of Mathematics, Oxford University Press 2005
[128] Harvard College v. Canada (Commissioner of Patents), [2002] S.C.C. 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraph 159. The court relied on the definitions of the term in the Oxford English Dictionary and the Grand Robert de la langue française
[129] Harvard College v. Canada (Commissioner of Patents) [2002] S.C.C. 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraphs 157-163
[130] Canada (Attorney General) v. Amazon.com, Inc. 2011 FCA 328 at paragraph 66
[131] Shell Oil v. Commissioner of Patents [(1982), 67 C.P.R. (2nd), 1 (S.C.C.)] at page 14
[132] Riello Canada, Inc. v. Lambert [(1986), 9 C.P.R. (3rd), 324 (F.C.T.D.)] citing at pages 335 and 336 Reynolds v. Herbert Smith & Co., Ltd. [(1902), 20 R.P.C., 123 (Ch.D.)]
[133] Harvard College v. Canada (Commissioner of Patents) [2002] S.C.C. 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraph 158
[134] Schlumberger Canada Ltd. V. Commissioner of Patents [(1981), 56 C.P.R. (2nd) 204 (F.C.A.)] at page 206
[135] Harvard College v. Canada (Commissioner of Patents) [2002] S.C.C. 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraphs 159 to 163
[136] Re Application No. 44,282 of Leubs (1971) C.D. 80 (relating to wood panels wherein the novelty lay in particular inscribed designs); Re Application No. 245,995 for a Townhouse building design [(1979) C.D. 605, 53 C.P.R. (2nd), 211 (P.A.B.)] (relating to architectural plans or designs); Re Application 040,799 of Cowan (1971) C.D. 79.; Lawson v. Commissioner of Patents [(1970), 62 C.P.R. (1st), 101 (Ex. Ct.)]
[139] Re Dixon Application No. 159, 204 [(1978 C.D. 493, 60 C.P.R. (2nd), 105 (P.A.B.)], the Commissioner cited with approval the conclusions reached in the UK cases Cooper’s Application [(1902) 19 R.P.C. 53] and Fishburn’s Application [(1940) 57 R.P.C. 245]
[140] Re Application No. 003,389 of N.V. Organon [(1973) C.D. 144, 15 C.P.R. (2nd), 253 (P.A.B)] at page 258
[141] Canada (Attorney General) v. Amazon.com, Inc. 2011 FCA 328 at paragraph 58
[142] Lawson v. Commissioner of Patents [(1970), 62 C.P.R. (1st), 101 (Ex. Ct.)] at page 115, in respect of “plans”
[143] Schlumberger Canada Ltd. V. Commissioner of Patents [(1981), 56 C.P.R. (2nd) 204 (F.C.A.)] at page 206
[145] Shire Biochem Inc. v. Minister of Health 2008 FC 538 at paragraph 61 ; Apotex Inc. v. Wellcome Foundation Ltd., [2002] 4 S.C.R. 153, 2002 SCC 77 at paragraph 37
[146] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraphs 24-27 and 33-37
[147] Eli Lilly and Company v. Apotex Inc. 2009 FC 991 at paragraph 397; Shire Biochem Inc. v. Minister of Health 2008 FC 538 at paragraph 75.
[148] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 25
[149] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraphs 33-37
[150] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraphs 24-46; Lundbeck Canada Inc. v. Ratiopharm Inc. 2009 FC 1102 at paragraph 69; Abbott Laboratories v. Minister of Health 2008 FC 1359 at paragraph 59 (aff’d 2009 FCA 94).
[151] Bristol-Myers Squibb Canada Co. v. Apotex Inc. 2009 FC 137 at paragraph 35
[152] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 25, citing Synthon B.V. v. SmithKline Beecham plc 2005 UKHL 59 at paragraph 32
[153] Schmuel Hershkovitz v. Tyco Safety Products Canada Ltd. 2009 FC 256 at paragraph 100; Shire Biochem Inc. v. Minister of Health 2008 FC 538 at paragraph 65
[154] Abbott Laboratories v. Minister of Health 2008 FC 1359 at paragraphs 59 and 60; Johnson & Johnson Inc. v. Boston Scientific Ltd. 2008 FC 552 at paragraph 309; this principle is also inherent in wording of subsection 28.2(1) of the Patent Act.
[155] Abbott Laboratories v. Minister of Health 2008 FC 1359 at paragraph 75 (aff’d 2009 FCA 94)
[156] Steel Co. of Canada Ltd. v. Sivaco Wire and Nail Co. [(1973), 11 C.P.R. (2nd), 153 (F.C.T.D.)] at page 190, citing General Tire & Rubber Co. v. Firestone Tyre & Rubber Co. Ltd. [1972] R.P.C. 464 at page 486; Abbott Laboratories v. Canada (Minister of Health) 2006 FCA 187 at paragraph 24, citing Smithkline Beecham PLC's (Paroxetine Methanesulfonate) Patent, [2005] UKHL 59 at paragraph 22, itself citing Merrell Dow Pharmaceuticals Inc v N.H. Norton & Co. Ltd. [1996] R.P.C. 76 at page 90
[157] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 27
[158] Free World Trust v. Électro Santé Inc. 2000 SCC 66 at paragraph 26 citing Consolboard Inc. c. MacMillan Bloedel (Saskatchewan) Ltd. [1981] 1 RCS 504 [(1981), 56 CPR (2nd), 145 (CSC)] per Dickson J. at p. 534
[159] See, e.g., Schmuel Hershkovitz v. Tyco Safety Products Canada Ltd. 2009 FC 256 at paragraph 105
[160] Reeves Bros. v. Toronto Quilting [(1978), 43 C.P.R. (2nd), 145 (F.C.T.D.)] at page 157, apparently relying on a proposition stated at least as early as Hill v. Evans (1869), 4 DeG. F. & J. 988, 45 E.R. 1195 at page 301. The continued relevance of the factors enumerated in Reeves Bros. was discussed in Johnson & Johnson Inc. v. Boston Scientific Ltd. 2008 FC 552 at paragraph 295.
[161] Lovell Manufacturing Co. v. Beatty Bros. Ltd. [(1962), 41 C.P.R. (1st), 18 (Ex. Ct.)] at page 45, citing Hill v. Evans (1869), 4 DeG. F. & J. 988, 45 E.R. 1195 at page 300
[162] Abbott Laboratories v. Canada (Minister of Health) 2006 FCA 187 at paragraphs 24 and 25; Eli Lilly and Company v. Apotex Inc. 2009 FC 991 at paragraph 397; AstraZeneca Canada Inc. v. Apotex Inc. 2010 FC 714 at paragraph 124
[163] Lightning Fastener Co. v. Colonial Fastener Co. [1933] S.C.R. 377 (affirming [1932] Ex. C.R. 101) at page 381.
[164] Shire Biochem Inc. v. Minister of Health 2008 FC 538 at paragraph 63
[165] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at para. 42
[166] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraphs 35 and 42
[167] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 at paragraphs 216-220
[168] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at para 42 citing Merrell Dow Pharmaceuticals Inc. v. H.N. Norton & Co. Ltd. (1995), [1996] R.P.C. 76 (H.L.) at p. 86
[169] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 citing Lux Traffic Controls Limited v. Pike Signals Limited, [1993] R.P.C. 107 (Pat. Ct.) at p.132
[170]. Wenzel Downhole Tools Ltd. v. National-Oilwell Canada Ltd 2012 FCA 333 at paragraphs 68 and 74
[171] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraph 42
[172] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraph 42; Gibney v. Ford Motor Co. of Canada [(1967), 35 Fox Pat. C. 143] at paragraph 61
[173] Abbott Laboratories v. Canada (Minister of Health) 2006 FCA 187 at paragraphs 23 to 25; Calgon Carbon Corporation v. North Bay (City) 2006 FC 1373 at paragraphs 114 to 136
[174]. See Metalliflex Limited v. Rodi & Wienenberger Aktiengesellschaft, [1961] S.C.R. 117
[175] Abbott Laboratories v. Minister of Health 2008 FC 1359 at paragraphs 69-73; Lundbeck Canada Inc. v. Ratiopharm Inc. 2009 FC 1102 at paragraphs 20, 118 and 136;
[176] Astrazeneca AB v. Apotex Inc. 2007 FC 688 at paragraphs 50-53
[177] The King v. American Optical Co. [(1950), 13 C.P.R. (1st), 87 (Ex. Ct.)] at pages 109-110, citing Clay v. Allcock & Co. (1906), 23 R.P.C. 745 at page 750
[178] Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 161, citing The King v. American Optical Co. [(1950), 13 C.P.R. (1st), 87 (Ex. Ct.)] at pages 109-110
[179] Commissioner of Patents v. Farbewerke Hoechst Aktiengesellschaft Vormals Meister Lucius & Bruning [1964] S.C.R. 49, [(1963), 41 C.P.R. (1st), 9 (S.C.C.)] at page 17
[180] The requirement codified in section 28.3 of the Patent Act that an invention not be obvious in view of certain prior art implies a requirement for ingenuity – see Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraphs 109-110; Canamould Extrusions Ltd. v. Driangle Inc. 2003 FCT 244 at paragraph 61 (rev’d on other grounds); Baker Petrolite Corp. v. Canwell Enviro Industries Ltd. 2001 FCT 889 at paragraphs 94-96 (rev’d on other grounds); Harvard College v. Canada (Commissioner of Patents) [(2000), 7 C.P.R. (4th), 1 (F.C.A.)] at paragraph 105 (rev’d on other grounds); Diversified Products v. Tye-Sil [(1991), 35 C.P.R. (3rd), 350 (F.C.A.)] at page 366.
[181] Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraphs 99, aff’d 2007 FCA 217. Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158.
[183] The requirement codified in section 28.3 of the Patent Act that an invention not be obvious in view of certain prior art implies a requirement for ingenuity – see Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraphs 109-110; Canamould Extrusions Ltd. v. Driangle Inc. 2003 FCT 244 at paragraph 61 (rev’d on other grounds); Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraphs 94-96 (rev’d on other grounds); Harvard College v. Canada (Commissioner of Patents) [(2000), 7 C.P.R. (4th), 1 (F.C.A.)] at paragraph 105 (rev’d on other grounds); Diversified Products v. Tye-Sil [(1991), 35 C.P.R. (3rd), 350 (F.C.A.)] at page 366.
[184] Beloit Canada Ltd. v. Valmet Oy [(1986), 8 C.P.R. (3rd), 289 (F.C.A.)] at page 293
[185] Diversified Products v. Tye-Sil [(1991), 35 C.P.R. (3rd), 350 (F.C.A.)] at page 366
[186] Xerox of Canada Ltd. v. IBM Canada Ltd. [(1977), 33 C.P.R. (2nd), 24 (F.C.T.D.)] at page 52, citing Samuel Parkes & Co. Ltd. v. Cocker Bros. Ltd. [(1929), 46 R.P.C. 241] at page 248.
[187] The King v. Uhlemann Optical Co. [(1951), 15 C.P.R. (1st), 99 (S.C.C.)] at pages 104-105; Wandscheer v. Sicard Ltd [1948] S.C.R. 1 [(1947), 8 C.P.R. (1st), 35 (S.C.C.)] at page 48; both cases citing Samuel Parkes & Co. Ltd. v. Cocker Bros. Ltd. [(1929), 46 R.P.C. 241] at page 248.
[188] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraphs 61-64; Janssen-Ortho Inc. v. Novopharm Limited 2007 FCA 217 at paragraph 25.
[189] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 67. The approach is based on that taken in Windsurfing International Inc. v. Tabur Marine (Great Britain) Ltd. [1985] R.P.C. 59 (C.A.) and refined in Pozzoli SPA v. BDMO SA [2007] EWCA Civ 588 and may be termed the Windsurfing/Pozzoli approach.
[190] Free World Trust v. Électro Santé Inc. 2000 SCC 66 at paragraph 44, quoting H.G. Fox from his Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed.] at page 184; Whirlpool Corp. v. Camco Inc. 2000 SCC 67 at paragraph 49, citing Lister v. Norton Brothers and Co. [(1886), 3 R.P.C. 199 (Ch.D.)] at page 203
[191] Free World Trust v. Électro Santé Inc. 2000 SCC 66 at paragraph 44
[192] Servier Canada Inc. v. Apotex Inc. 2008 FC 825 at paragraph 254
[193] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 37
[194] Allergan Inc v Canada (Health) and Cobalt Pharmaceuticals, 2014 FC 566 at paragraph 25; Allergan Inc v Canada (Health) and Apotex Inc, 2014 FC 567 at paragraph 25
[195] Janssen-Ortho Inc. v. Novopharm Ltd. 2006 FC 1234 at paragraph 113, aff’d 2007 FCA 217 at paragraph 25; these factors are considered to remain relevant in view of the guidance of the Supreme Court in Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61.
[196] Canadian Gypsum Co. v. Gypsum, Lime & Alabastine, Canada Ltd. [1931] Ex. C.R. 180 at paragraph 12
[197] Sanofi‑Aventis Canada Inc. v. Ratiopharm Inc. 2010 FC 230 at paragraphs 83-87; Commissioner’s Decision 1304 at paragraph 43
[198] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 68
[199] Wenzel Downhole Tools Ltd. v. National-Oilwell Canada Ltd., 2011 FC 1323 at paragraphs 193 to 197 where the obvious-to-try test was applied to downhole drilling equipment. Comments on the appropriateness of the test were made on appeal (see 2012 FCA 333) at paragraphs 91 to 108, especially paragraph 95.
[200] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 59-69, especially at 59, 64, 68 and 69; Sanofi-Aventis v. Apotex Inc., 2013 FCA 186 at paragraphs 74-80
[201] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 68
[202]. The King v. American Optical Co. [(1950), 13 C.P.R. (1st), 87 (Ex. Ct.)] at page 98
[203] Schmuel Hershkovitz v. Tyco Safety Products Canada Ltd. 2009 FC 256 at paragraph 148 referring to R.H. Barrigar, Canadian Patent Act Annotated, 2nd ed. (Aurora: Canada Law Book, 2008) at PA-28.11-12; Domtar Ltd. v. McMillan Bloedel Packaging Ltd. (1977), 33 C.P.R. (2d) 182 at 189-91 (F.C.T.D.), affirmed (1978), 41 C.P.R. (2d) 182 (F.C.A.).
[204]. Crila Plastic Industries Ltd. v. Ninety-eight Plastic Trim Ltd. 18 C.P.R. (3d) 1 at pages 1 and 7 to 9, affirming 10 C.P.R. (3d) 226, referring to Domtar Ltd. v. McMillan Bloedel Packaging Ltd. (1977), 33 C.P.R. (2d) 182 at 189-91 (F.C.T.D.), affirmed (1978), 41 C.P.R. (2d) 182 (F.C.A.).
[205] Visirecord of Canada Ltd. v. Malton [1958] Ex. C.R. 116 at paragraph 59, citing Lightning Fastener Company Limited v. Colonial Fastener Company, Limited [1932] Ex. C.R. 101 at page 106
[206] Visirecord of Canada Ltd. v. Malton [1958] Ex. C.R. 116 at paragraph 60, citing Lowe-Martin Company Ltd. v. Office Specialty Manufacturing Company Ltd. [1930] Ex. C.R. 181 at page 187
[207] Johnson Controls, Inc. v. Varta Batteries Ltd. [(1984), 80 C.P.R. (2nd), 1 (F.C.A.)] at pages 12-13
[208] Visirecord of Canada Ltd. v. Malton [1958] Ex. C.R. 116 at paragraph 61, citing The Railroad Supply Co. v. The Elyria Iron and Steel Co. [1917] Patent Office Gaz. (U.S.) vol. 239, at page 658
[209] Visirecord of Canada Ltd. v. Malton [1958] Ex. C.R. 116 at paragraph 62, citing Helson v. Dominion Dustless Sweepers Co. Limited (1923), 23 O.W.N. 597 at page 598
[211] Uview Ultraviolet Systems Inc. v. Brasscorp Ltd. 2009 FC 58 at paragraph 224.
[212] Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 168
[213] Whirlpool Corp. v. Camco Inc. 2000 SCC 67 at paragraph 63-67
[214] Abbott Laboratories v. The Minister of Health 2009 FC 648 at paragraph 187, referring to Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 169, itself referring to Commissioner of Patents v. Farbewerke Hoechst Aktiengesellschaft Vormals Meister Lucius & Bruning [1964] S.C.R. 49, [(1963), 41 C.P.R. (1st), 9 (S.C.C.)] at page 13
[215] Commissioner of Patents v. Farbewerke Hoechst Aktiengesellschaft Vormals Meister Lucius & Bruning [1964] S.C.R. 49, [(1963), 41 C.P.R. (1st), 9 (S.C.C.)] at page 13.
[216] GlaxoSmithKline Inc. v. Apotex Inc. 2003 FCT 687 at paragraphs 89‑91
[217] GlaxoSmithKline Inc. v. Apotex Inc. 2003 FCT 687 at paragraph 37.
[218] GlaxoSmithKline Inc. v. Apotex Inc. 2003 FCT 687 at paragraphs 87‑91; Bayer Inc. v. Canada (Minister of National Health and Welfare) 154 F.T.R [(1998), 82 C.P.R. (3rd), 359 (F.C.T.D.), aff'd (2000), 6 C.P.R. (4th), 285 (F.C.A.] at paragraph 33. See also Apotex Inc. v. Merck & Co. 2006 FCA 323 at paragraph 49.
[219] Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 169
[220] I.G. Farbenindustrie A.G.'s Patents [(1930), 47 R.P.C. 289] at pages 322-323; these criteria appear to have been endorsed in Canada at least as early as 1947 in Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1947), 12 C.P.R. (1st), 102 (Ex.Ct.)] at pages 163-164) and were endorsed by the Supreme Court in Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 10.
[221] GlaxoSmithKline Inc. v. Pharmascience Inc. 2008 FC 593 at paragraph 70 and at paragraph 51 with reference to Dreyfus and Others Application [(1945), 62 R.P.C. 125 (H.L.)] at page 133; I.G. Farbenindustrie A.G.'s Patents [(1930), 47 R.P.C. 289] at page 327
[222] Eli Lilly Canada Inc. v. Novopharm Limited 2010 FCA 197 at paragraphs 27, 30; Ratiopharm Inc. v. Pfizer Limited 2009 FC 711 at paragraph 175, aff’d 2010 FCA 204 at paragraph 33
[223] Pfizer Canada Inc. v. Canada 2006 FCA 214 at paragraph 4
[224]. Pfizer Canada Inc. v. Ranbaxy Laboratories Limited 2008 FCA 108 at paragraph 59; Eli Lilly Canada Inc. v. Apotex Inc. 2007 FC 455 at paragraph 89
[225] I.G. Farbenindustrie A.G.'s Patents [(1930), 47 R.P.C. 289] at page 323
[226] see, e.g., Eli Lilly Canada Inc. v. Novopharm Limited 2009 FC 235 at paragraph 100; Eli Lilly Canada Inc. v. Novopharm Ltd. 2007 FC 596 at paragraph 162; Ratiopharm Inc. v. Pfizer Limited 2009 FC 711 at paragraph 179;
[227] Ratiopharm Inc. v. Pfizer Limited 2009 FC 711 at paragraph 175, aff’d 2010 FCA 204 at paragraphs 27-28
[228] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 9; I.G. Farbenindustrie A.G.'s Patents [(1930), 47 R.P.C. 289] at page 321
[229] Pfizer Limited v. Ratiopharm Inc. 2010 FCA 204 at paragraphs 27-28
[230] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 57
[231] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 52
[232] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 56, citing Re Application of Abitibi Co. (1982), 62 C.P.R. (2d) 81, (Patent Appeal Board and Commissioner of Patents), at p. 91
[233] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 54
[234] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 53
[235] AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 55
[236] Teva Canada Ltd. v. Pfizer Canada Inc. 2012 SCC 60 at paragraph 40; AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 58
[237] Apotex Inc. v. Wellcome Foundation Ltd. 2002 SCC 77 at paragraph 46; AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraphs 55-56
[238] Feherguard Products Ltd. v. Rocky’s of BC Leisure Ltd. [(1995), 60 C.P.R. (3rd), 512 (F.C.A.)] at pages 516 to 517.
[239] Metalliflex Ltd. v. Rodi & Wienenberger AG [1961] SCR 117 & [(1960), 35 C.P.R. (1st), 49 (S.C.C.)] at pages 53-54
[240] Re Application No. 003,389 of N.V. Organon [(1973) C.D. 144, 15 C.P.R. (2nd), 253 (P.A.B)] at page 258
[241] Pioneer Hi-Bred Ltd. v. Canada (Commissioner of Patents) [(1989), 25 C.P.R. (3rd), 257 (S.C.C.)] at page 270.
[242] Re Application for Patent Containing Claims that Read on Mental Steps [(1972) C.D. XXX, 23 C.P.R. (2nd), 93]; Re Application 269,230 of Itek Corporation (1981) C.D. 896
[243] Apotex Inc. v. Wellcome Foundation Ltd. 2002 SCC 77 at paragraph 46
[244] Apotex Inc. v. Wellcome Foundation Ltd. 2002 SCC 77 at paragraph 46
[245] Pfizer Canada Inc. v. Apotex Inc. 2007 FC 26 at paragraph 70; aff’d 2007 FCA 195
[246] Pfizer Canada Inc. v. Novopharm Limited 2009 FC 638 at paragraph 82, aff’d 2010 FCA 242 at paragraph 82, aff’d 2012 SCC 60 at paragraph 40
[247] Bell Helicopter Textron Canada Ltd. v. Eurocopter 2013 FCA 219 at paragraphs 48-51 and 135-162
[248] Apotex Inc. v. Wellcome Foundation Ltd. 2002 SCC 77 at paragraph 70
[249] Aventis Pharma Inc. v. Apotex Inc. 2005 FC 1283, 43 C.P.R. (4th) 161 at paragraph 164; aff’d on this point 2006 FCA 64, 46 C.P.R. (4th) at paragraph 30; AstraZeneca Canada Inc. v. Apotex Inc., 2017 SCC 36 at paragraph 46
[250] Apotex Inc. v. Wellcome Foundation Ltd. 2002 SCC 77 at paragraph 69
[251] Monsanto Co. v. Commissioner of Patents [(1979), 42 C.P.R. (2nd), 161 (S.C.C.)] at page 176, citing Olin Mathieson Chemical Corp. et al. v. Biorex Laboratories Ltd. et al. [1970] R.P.C. 157
[252] Pfizer Canada Inc. v. Canada (Minister of Health) 2007 FCA 209 at paragraph 152\
[253] Pfizer Canada Inc. v. Apotex Inc. 2007 FC 26 at paragraph 70; aff’d 2007 FCA 195
[254] Eli Lilly Canada Inc. V. Apotex Inc. 2009 FCA 97 at paragraphs 10 to 18; Eli Lilly Canada Inc. v. Novopharm Limited 2009 FC 235 at paragraph 101; Servier Canada v. Apotex Inc. 2008 FC 825 at paragraph 99
[255] Apotex Inc. v. Pfizer Canada Inc. 2011 FCA 236 at paragraph 52
[256] Eli Lilly Canada Inc. v. Apotex Inc. 2008 FC 142 at paragraphs 163-164; Eli Lilly Canada Inc. V. Apotex Inc. 2009 FCA 97 at paragraph 12
[257] Eli Lilly v. Apotex Inc. 2009 FCA 97 at paragraph 18; this requirement extends equally to any factual basis needed to support a sound prediction of an advantage possessed by a selection from a broader group, see Pfizer Canada Inc. v. Canada (Minister of Health) 2008 FC 500 at paragraph 97 and GlaxoSmithKline Inc. v. Pharmascience Inc. 2008 FC 593 at paragraph 71
[258] Eli Lilly Canada Inc. v. Novopharm Ltd. 2010 FCA 197 at paragraph 120
[259] Re: Application No. 139,256 (Patent No. 1,029,723) [1977] 51 C.P.R. (2d) 95 at 103; Re Application No. 315,073 [(1981) C.D. 904]; Re Application No. 2,313,707 [(2013) C.D. 1353]
[260] More information on free text and language-dependent free text can be found in paragraphs 85-88 of the PCT sequence listing standard, WIPO Standard 26 (ST.26).
[261] While the analysis is similar, the requirements providing authority differ. Regularly-filed Canadian (non-PCT) applications must meet the requirements of section 91 of the Patent Rules, which indicates that the matter hypothetically added to the parent application must comply with all of section 38.2 of the Patent Act, with exception to subsection (4). PCT national phase applications must meet the requirements provided in section 155.7 of the Patent Rules, in which hypothetical amendments to the parent application must comply with subsection 38.2(1), without taking into account subsection (4). Subsection 38.2(1) of the Patent Act permits amendments subject to subsections 38.2(2)-(4). Subsections 38.2(3) and 38.2(3.1)(a) of the Act do not apply to PCT national phase applications, per section 159 of the Patent Rules. Accordingly, equivalents of these provisions are included in section 155.7 by reference to subsections 155.6(1) and 155.6(3) of the Patent Rules (by the broad, over-arching reference to section 155.6 of the Rules). As 155.6(4) provides similar requirements to 38.2(4) it is also not taken into account for the analysis.
[262] While the analysis is similar, the requirements providing authority differ. Amendments to regularly-filed Canadian (non-PCT) applications must meet the requirements of subsection 38.2(3.1) of the Patent Act. As paragraph 38.2(1)(a) of the Act does not apply to PCT national phase applications (according to section 159 of the Patent Rules), it cannot be applied. Instead, the provisions of subsection 155.6(3) of the Rules permits an equivalent provision for PCT applications, while requiring further analysis for applications filed in a language other than English or French. Paragraph 38.2(3.1)(b) of the Act still applies to PCT national phase applications.
[263] Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 168 referring to “the well-known rule that only one patent may issue for a given invention”; and Teva Canada Ltd. v. Pfizer Canada Inc. 2012 SCC 60 at paragraph 58 affirming that “a patent shall be granted for one invention only.”
[264] Or of a divisional application to cover several additional inventions disclosed in the parent application, or of one or several divisional applications each to cover one of several additional inventions disclosed in the parent application.
[265] Merck & Co., Inc. v. Apotex Inc. 2006 FC 524 at paragraph 203. Hughes J. also noted at paragraph 197 that “[d]uring the pendency of an application or several applications, the procedures to be followed are the prerogative of the Patent Office”
.
[266] Libby‑Owens‑Ford Glass Co. v. Ford Motor Co. [(1970), 62 C.P.R. (1st), 223 (S.C.C.)] at pages 230-231, Ciba-Geigy AG v. Commissioner of Patents [(1982), 65 C.P.R. (2nd), 73 (F.C.A.)] at page 79
[267] Sociéte des Usines Chimiques Rhone‑Poulenc et al. v. Jules R. Gilbert Ltd. [1966] Ex. C.R. 59 at paragraphs 6-8
[268] In view of this, some content in this chapter mirrors or has been adapted from text found in the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018).
[269] Article 27(1) PCT states: No national law shall require compliance with requirements relating to the form or contents of the international application different from or additional to those which are provided for in this Treaty and the Regulations.
[270] PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018).
[271] Teva Canada Ltd. v. Pfizer Canada Inc. 2012 SCC 60 at paragraph 64
[272] For an example of corresponding elements, see section 10.29 of the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018).
[273] This example is adapted from the example provided in section 10.23 of the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018).
[274] This example is adapted from the example provided in section 10.26 of the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018).
[275] The conclusion reached in section 10.43 of the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018) can be understood in this light, presuming that a single line of reasoning cannot soundly predict why the various classes of herbicide B work with A to achieve the inventive result.
[276] See also the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2011) at 10.42.
[277] The conclusion reached in section 10.58 of the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018) can be understood in this light, since compounds X, Y and Z do not share a structural feature responsible for their activity. It must be presumed that X, Y and Z are not members of a recognised class of compounds.
[278] Due regard should be given to the nature of the synthesis in performing this evaluation. The relationship of the structure of an intermediate to the final product will be quite different in, for example, a convergent synthesis than in a divergent synthesis, or in a ring-closing or rearrangement reaction than in an addition reaction. See also the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018) at 10.18(f).
[279] See the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018) at 10.18(e).
[280] This example is loosely based on the PCT International Search and Preliminary Examination Guidelines published by the World Intellectual Property Organization (Geneva, 2018) at 10.47, which provides specific chemical structures to illustrate the same point.
[281] A method for preparing a product would usually be considered to render the product it produces obvious, and there could consequently be an appearance of double-patenting if claims 2 and 3 appeared in different applications.
[282] Consolboard Inc. v. Macmillan Bloedel (Saskatchewan) Ltd. [(1981), 56 C.P.R. (2nd), 145 (S.C.C.)] at page 169
[283] Source code for computer programs may, however, be subject to the protection of the Copyright Act as a literary work.
[284] Schlumberger Canada Ltd. v. Commissioner of Patents [(1981), 56 C.P.R. (2nd),204 (F.C.A.)] at page 206
[285] i.e. provide a technological solution to a technological problem
[286] Re Application for Patent Containing Claims that Read on Mental Steps [(1972), 23 C.P.R. (2nd), 93] ; Re Application 269,230 of Itek Corporation (1981) C.D. 896
[287] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraphs [35] and [42]
[288] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 at paragraphs [216] to [220]
[289] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 citing Merrell Dow Pharmaceuticals Inc. v. H.N. Norton & Co. Ltd. (1995), [1996] R.P.C. 76 (H.L.) at p. 86
[290] Bauer Hockey Corp. v. Easton Sports Canada Inc. 2010 FC 361 citing Lux Traffic Controls Limited v. Pike Signals Limited, [1993] R.P.C. 107 (Pat. Ct.) at p.132
[291] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraph [42]
[292] Canwell Enviro Industries Ltd. v. Baker Petrolite Corp. 2002 FCA 158 at paragraphs [41]-[42]
[293] see, e.g., the comments in Re Application 2,349,479 of U-Haul International Inc. (2010) C.D. 1298 at paragraphs [37] to [42]
[295] Office Practice Regarding Signals C.P.O.R. Vol. 135, No. 33, August 14, 2007
[296] A signal is considered to be propagating even if it is moving in a closed loop.
[297] United Nations Convention on Biological Diversity, Article 2. Use of Terms, 1992 [(http://www.cbd.int/convention/text/); retrieved: 31 October 2011]
[298] Harvard College v. Canada (Commissioner of Patents), 2002 SCC 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraphs 197-199
[300] Harvard College v. Canada (Commissioner of Patents), 2002 SCC 76; [(2002), 21 C.P.R. (4th), 417 (S.C.C.)] at paragraphs 153-166
[301] For the purposes herein, a totipotent stem cell is defined as a cell capable of giving rise to all types of differentiated cells found in an organism, as well as the supporting extra-embryonic structures of the placenta. A single totipotent cell could, by division in utero, reproduce the whole organism. This definition is adopted from that provided in the Glossary on the National Institutes of Health, Stem Cell Information website, https://stemcells.nih.gov/ , retrieved November 2014
[302] For the purposes herein, embryonic stem cells are defined as primitive (undifferentiated) cells that are derived from preimplantation-stage embryos, are capable of dividing without differentiating for a prolonged period in culture, and are known to develop into cells and tissues of the three primary germ layers. Multipotent cells have the ability to develop into more than one cell type of the body. Pluripotent stem cells are capable of differentiating into all tissues of an organism, but are not alone capable of sustaining full organismal development. These definitions are adopted from those provided in the Glossary on the National Institutes of Health Stem Cell Information website, https://stemcells.nih.gov/, retrieved November 2014
[303] Monsanto Canada Inc. v. Schmeiser, 2004 SCC 34; [(2004), 31 C.P.R. (4th), 161 (S.C.C.)] at paragraph 17
[305] Pioneer Hi-Bred Ltd. v. Canada (Commissioner of Patents), [1989] 1 S.C.R. 1623 [(1989), 25 C.P.R. (3rd), 257(S.C.C.)] at pages 263-265 (cited to C.P.R.)
[306] Tennessee Eastman v. Commissioner of Patents [(1972), 8 C.P.R. (2nd), 203 (S.C.C.)]; Imperial Chemical Industries Ltd. v. Commissioner of Patents [(1986), 9 C.P.R. (3rd), 289 (F.C.A.)]
[307] This conclusion is inferred from the decision in Re Application 319,105 of Boehringer Mannheim G.m.b.H. (1987) C.D. 1108, allowing a diagnostic method involving the removal of blood from the body
[309] Re Application No. 532,566 of General Hospital Corporation (1996) C.D. 1209; Re Application No. 559,960 of Senentek (1997) C.D. 1213
[310] Re Application No. 003,389 of N.V. Organon [(1973) C.D. 144, 15 C.P.R. (2nd), 253 (P.A.B.)]; Re Application for Patent of Goldenberg [(1988) C.D. 1119, 22 C.P.R. (3rd), 159 (P.A.B.)]
[312] Axcan Pharma Inc. v. Pharmascience Inc., [2006] FC 527 [(2006), 50 C.P.R. (4th), 321 (F.C.)]
[313] Re Application No. 003,772 of Ijzerman (1975) C.D. 254; Merck & Co. v. Apotex Inc. [2005] FC 755 [(2005), 41 C.P.R. (4th), 35 (F.C.)]
[315]. The term “analyte” is used broadly herein to mean a chemical substance or biomarker that is the subject of analysis.
[316] AstraZeneca Canada Inc. v. Apotex Inc., 2010 FC 714 at paragraph 33; Wenzel Downhole Tools Ltd. v National-Oilwell Canada Ltd., 2011 FC 1323 at paragraph 61; Jay-Lor International Inc. v. Penta Farm Systems Ltd., 2007 FC 358 at paragraph 55; Sanofi-Aventis Canada Inc. v. Apotex, 2009 FC 676 at paragraph 128; Merck & Co. Inc. v. Apotex Inc., 2010 FC 1265 at paragraph 86
[317] To better illustrate, consider a situation where the measurement of analyte X had been routinely performed in urine samples (i.e., the measurement of X in urine was CGK to the POSITA) but in the instant application it is apparent that the inventors have instead performed the measurement of X in saliva. Although the means by which X is measured is the same (e.g., chromatography), using a saliva sample instead of a urine sample would not represent the standard sample source for measuring X and thus would be “non-standard to that means”.
[318] For example, consider a situation where it was routine to test for the presence of analyte X after exposure to environmental hazard Z (i.e., the measurement of X after exposure to Z was CGK to the POSITA) but in the instant application the testing for analyte X was performed precisely 36-48 hours post-exposure. Although the assay used to detect X is the same, in this case performing the assay within a window of 36-48 hours post-exposure is not routine and thus would be “non-standard to that means”.
[319] Radio Corporation of America v. Raytheon Manufacturing Co. [(1957), 27 C.P.R. (1st), 1 (Ex.Ct.)] at page 14
[320] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1949), 12 C.P.R. (1st), 99 at page 111]; the cited passage has been referred to more recently in, e.g., Baker Petrolite Corp. v. Canwell Enviro-Industries Ltd. 2001 FCT 889 [(2001), 13 C.P.R. (4th), 193 (F.C.T.D.)] (rev’d on other grounds) and 671905 Alberta Inc. v. Q’Max Solutions Inc. 2001 FCT 888 [(2001), 14 C.P.R. (4th), 129 (F.C.T.D.)] (varied [(2003), 27 C.P.R. (4th), 385 (F.C.A.)]). Minerals Separation was referred to in both Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [1981] 1 S.C.R. 504 at page 520 and Pioneer Hi-Bred Ltd. v. Canada (Commissioner of Patents), [1989] 1 S.C.R. 1623 [(1989), 25 C.P.R. (3rd), 257(S.C.C.) at page 268] as in a general sense setting out the requirements of a sufficient disclosure.
[321] Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [1981] 1 S.C.R. 504 at page 517, Dickson J. quoting H.G. Fox from his Canadian Law and Practice Relating to Letters Patent for Inventions [(1969), 4th Ed.]
[323] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1949), 12 C.P.R. (1st), 99 at page 111]; this passage endorsed in Consolboard Inc. v. MacMillan Bloedel (Saskatchewan) Ltd. [1981] 1 S.C.R. 504 at page 520
[325] Reeck, Gerald et al., “ ‘Homology’ in proteins and nucleic acids: A terminology muddle and a way out of it” (1987), 50 Science 667
[326] Altschul, S. et al., “Basic Local Alignment Search Tool” (1990), 215 Journal of Molecular Biology 403
[327] Janssen-Ortho Inc. v. Novopharm Limited, 2006 FC 1234 [(2006), 57 C.P.R. (4th), 6 (F.C.)] at paragraph 99, aff’d 2007 FCA 217 [(2007), 59 C.P.R. (4th), 116 (F.C.A.)]. The requirement of section 28.3 has been variously described by the courts as one of “ingenuity”, “inventive ingenuity”, “invention”, “inventiveness”, and “non-obviousness”. These terms can be used more or less interchangeably to describe the requirement codified in s.28.3.
[328] Apotex Inc. v. Sanofi-Synthelabo Canada Inc. 2008 SCC 61 at paragraph 67
[329] Section 46 of the Patent Rules indicates that the text matter in the abstract, the description, the drawings and the claims, other than any text matter contained in a sequence listing, must be entirely in English or entirely in French.
[330] For applications filed prior to June 2, 2007, see section 194 of the Patent Rules.
[331] The feature table of a sequence contains information on the location and roles of various regions of interest within a particular sequence and is required for every sequence except intentionally skipped sequences [see 23.05.07c]. Paragraphs 60 et seq of the PCT Sequence Listing Standard provide more information on feature tables and mandatory elements thereof.
[332] Minerals Separation North American Corp. v. Noranda Mines, Ltd. [(1949), 12 C.P.R. (1st), 99 at page 111]
[333] Pioneer Hi-Bred Ltd. v Canada (Commissioner of Patents), 1989 S.C.R. 1623 [(1989), 25 C.P.R. (3rd), 257(S.C.C.) at page 271]
[334] Re Application of Abitibi Co. [(1982) C.D. 933, 62 C.P.R. (2nd), 81 (P.A.B.)]; Re Application No. 291,870 of Connaught Laboratories [(1982) C.D. 962]
[335] Cobalt Pharmaceuticals Company v. Bayer Inc., 2015 FCA 116 at paragraph 67 and Teva Canada Ltd. v. Pfizer Canada Inc., 2012 SCC 60 at paragraph 90
[336] Re Application No. 2,451,493 (2016) C.D. 1398 at paragraph 22 citing Novartis Pharmaceuticals Canada Inc. v. Teva Canada Ltd., 2013 FC 283
[337] Re Application No. 2,451,493 (2016) C.D. 1398 citing Re Immunex Corporation Patent Application No. 583,988 [(2010) C.D. 1302, 89 C.P.R. (4th) 34 (P.A.B.)] at paragraph 67-68
[338] Re Genentech Inc. Patent Application No. 2,407,304 [(2010) C.D. 1307, 92 C.P.R. (4th) 241 (P.A.B.) at paragraph 68]
[339] Re Genentech Inc. Patent Application No. 2,407,304 [(2010) C.D. 1307, 92 C.P.R. (4th) 241 (P.A.B.) at paragraph 67]
[340] Re Immunex Corporation Patent Application No. 583,988 [(2010) C.D. 1302, 89 C.P.R. (4th) 34 (P.A.B.) at paragraph 69]
[341] Apotex Inc. v. Pfizer Canada Inc. 2014 FCA 250 at paragraph 64
[342] Apotex Inc. v. Pfizer Canada Inc. 2014 FCA 250 at paragraph 64, citing Eli Lilly Canada Inc. v. Novopharm Limited 2010 FCA 197 at paragraph 76
[343] Lundbeck Canada Inc. v. Ratiopharm Inc., 2009 FC 1102, 79 C.P.R. (4th) 243 at paragraphs 228-229
[344] Lundbeck Canada Inc. v. Ratiopharm Inc., 2009 FC 1102, 79 C.P.R. (4th) 243
[345] Minerals Separation North American Corp v Noranda Mines Ltd [(1949), 12 C.P.R. (1st), 99 at page 111]
[346] Applicable only for applications for which examination is requested on or after October 3, 2022.
[347] Applicable only for applications for which examination is requested on or after October 3, 2022.
[348] A further report would not be written, for example, solely to advise the applicant that the next report may be made final, where the report otherwise simply reiterates the arguments presented in the previous report.
[349] A further report may not be necessary, for example, where the examiner has previously identified a defect as a non-compliance with one section of the Act or Rules, but later realises that for the same or substantially the same reasons the defect in question results in non-compliance with a further section of the Act or Rules or that the defect should have been identified as non-compliance with a different section of the Act and Rules than that previously identified.
[350] If the examiner had previously identified something as belonging to the common general knowledge, and the applicant had acknowledged this in correspondence, it would not be necessary to further substantiate that it is, in fact, common general knowledge. Similarly, if a claim with five elements was identified as being anticipated in view of a document D1, and the applicant agrees that D1 teaches four of the five claimed elements, it is not necessary to elaborate on those features in the reasons for the rejection; the point of disagreement is whether D1 discloses the fifth element.
[351] Despite the fact that any apparent defects will be identified, a review begins with the presumption that the search and examination prior to the review stage is complete and comprehensive.
[352] The PAB was created in a “Notice to the Patent Profession” (re: creation of the PAB, general guidelines, and hearing procedure) C.P.O.R., Aug. 4, 1970
[353] Canada. (2013). Regulatory Impact Analysis Statement, Rules Amending the Patent Rules. In Canada Gazette, Part II, Vol. 147, No. 26, 18 December 2013.
[355] Monsanto Co. v. Canada (Commissioner of Patents) (1976), 28 CPR (2d) 118 at 119
[370] Hershkovitz v. Tyco Safety Products Canada Ltd., 2009 FC 256 at para 93; Canadian Celanese Ltd. v. B.V.D. Co. Ltd., [1939] 2 DLR 289 at 294
[378] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at para 254
[379] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at 653; Bergeon v. DeKermor, [1927] 2 DLR 99 at para 38
[380] Commissioner’s Decision #1330 at para 43-44
[381] Curl-Master Manufacturing Co. Ltd. v. Atlas Brush Ltd. (1967), 52 CPR 51 at para 74
[382] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at para 254
[383] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at para 255; Burton Parsons Chemicals Inc. v. Hewlett-Packard (Canada) Ltd. (1974), 17 CPR (2d) 97 at 107; Creations 2000 Inc v. Canper Industrial Products Ltd. (1988), 22 CPR (3d) 389 at 406 aff’d 34 CPR (3d) 178
[384] Commissioner’s Decision #1289 at para 67-68; Commissioner’s Decision # 1279 at para 11, 14
[385] Commissioner’s Decision # 1297 at para 26, 44
[386] Commissioner’s Decision #1289 at para 46
[387] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649
[388] Paul Moore Co. Ltd. v. Commissioner of Patents (1979), 46 CPR (2d) 5 at 10
[389] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at 654; Mobil Oil Corp v. Hercules Canada Inc. (1994), 57 CPR (3d) 488 at para 498, 499 rev’d on other grounds 63 CPR (3d) 473; Commissioner’s Decision # 1173 at para 8
[390] Mobil Oil Corp v. Hercules Canada Inc. (1994), 57 CPR (3d) 488 at para 499
[391] Commissioner’s Decision #1289 at para. 41; Commissioner’s Decision #1333 at para. 26
[392] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at para 654; Curl-Master Manufacturing Co. Ltd. v. Atlas Brush Ltd. (1967), 52 CPR 51 at para 68-69
[393] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at para 654
[394] Commissioner’s Decision #134 at 5; Commissioner’s Decision #326 at 9; Commissioner’s Decision #420 at 1; Commissioner’s Decision #783 at 4-5; Commissioner’s Decision #906 at 10 Commissioner’s Decision #1148 at 17; Commissioner’s Decision #1186 at 5
[395] Burton Parsons Chemicals Inc. v. Hewlett-Packard (Canada) Ltd. (1974), 17 CPR (2d) 97 at para 108
[396] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at para 259
[397] It may be possible to have a patent reissued on the grounds of prior art discovered after its grant, as there is the presumption that a patentee would want patent claims whose subject matter does not overlap with any prior disclosure.
[398] Curl-Master Manufacturing Co. Ltd. v. Atlas Brush Ltd. (1967), 52 CPR 51 at para 68, 70-71; Mobil Oil Corp v. Hercules Canada Inc. (1994), 57 CPR (3d) 488 at para 501; Commissioner’s Decision #1289 at para 21
[399] Curl-Master Manufacturing Co. Ltd. v. Atlas Brush Ltd. (1967), 52 CPR 51 at 68, 70-71; Mobil Oil Corp v. Hercules Canada Inc. (1994), 57 CPR (3d) 488 at para 501
[400] Curl-Master Manufacturing Co. Ltd. v. Atlas Brush Ltd. (1967), 52 CPR 51 at para 70-71
[401] Commissioner’s Decision #56 at para 7
[402] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at para 659
[403] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at 254
[404] Commissioner’s Decision #1093 at para 6-7; Commissioner’s Decision #1173 at para 3
[406] Northern Electric Company Ltd. v. Photo Sound Corporation, [1936] SCR 649 at para 652-653; Creations 2000 Inc v. Canper Industrial Products Ltd. (1988), 22 CPR (3d) 389 at 406 aff’d 34 CPR (3d) 178 at para 406
[408] Farbwerke Hoechst Aktiengesellschaft vormals Meister Lucius & Bruning v. Commissioner of Patents (1966), 50 CPR 220 at para 245-46
[411] The Office will not acknowledge payment of maintenance fees paid before or on the maintenance fee due date.